A Comparative Study of Approved Drugs for SARS-CoV-2 by Molecular Docking
Main Article Content
Abstract
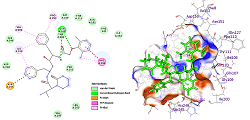
SARS-CoV-2, a new type of Coronavirus, has affected more millions of people worldwide. From the spread of this infection, many studies related to this virus and drug designing for the treatment have been started. Most of the studies target the SARS-CoV-2 main protease, spike protein of SASR-CoV-2, and some are targeting the human furin protease. In the current work, we chose the clinically used drug molecules remdesivir, favipiravir, lopinavir, hydroxychloroquine, and chloroquine onto the target protein SARS-CoV-2 main protease. Docking studies were performed using Arguslab, while Discovery Studio collected 2D and 3D pose views with the crystal structure of COVID-19 main protease in complex with an inhibitor N3 with PDB ID 6LU7. Computational studies reveal that all ligands provided good binding affinities towards the target protein. Among all the chosen drugs, lopinavir showed the highest docking score of -11.75 kcal/mol. The results from this molecular docking study encourage the use of lopinavir as the first-line treatment drug due to its highest binding affinity.
Downloads
Article Details
Authors continue to retain the copyright to the article if the article is published in the Journal of Molecular Docking. They will also retain the publishing rights to the article without any restrictions.
Authors who publish with this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal, right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles of work (eg, post-institutional repository) or publish it in a book, with acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process, as can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.
References
2. Waals AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281-92. doi:10.1016/j.cell.2020.02.058
3. Wu C, Zheng M, Yang Y, Gu X, Yang K, Li M, et al. Furin: A Potential Therapeutic Target for COVID-19. iScience. 2020;23(10):101642. doi:10.1016/j.isci.2020.101642
4. Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56(2):106054. doi:10.1016/j.ijantimicag.2020.106054
5. Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71-6. doi:10.1016/j.ijsu.2020.02.034
6. Dhama K, Sharun K, Tiwari R, Dadar M, Malik YS, Singh KP, et ap. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;16(6):1232-8. doi:10.1080/21645515.2020.1735227
7. Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent Sci. 2020;6(3):315-31. doi:10.1021/acscentsci.0c00272
8. Poduri R, Joshi G, Jagadeesh G. Drugs targeting various stages of the SARS-CoV-2 life cycle: Exploring promising drugs for the treatment of Covid-19. Cell Signal. 2020;74:109721. doi:10.1016/j.cellsig.2020.109721
9. Sharifkashani S, Bafrani MA, Khaboushan AS, Pirzadeh M, Kheirandish A, Bali HY, et al. Angiotensin-converting enzyme 2 (ACE2) receptor and SARS-CoV-2: Potential therapeutic targeting. Eur J Pharmacol. 2020;884:173455. doi:10.1016/j.ejphar.2020.173455
10. Nishima W, Kulik M. Full-Length Computational Model of the SARS-CoV-2 Spike Protein and Its Implications for a Viral Membrane Fusion Mechanism. Viruses. 2021;13(6):1126. doi:10.3390/v13061126
11. Voit K, Timmermann C, Steger F. Medication of Hydroxychloroquine, Remdesivir and Convalescent Plasma during the COVID-19 Pandemic in Germany—An Ethical Analysis. Int J Environ Res Public Health. 2021;18(11):5685. doi:10.3390/ijerph18115685
12. Yavuz SŞ, Ünal S. Antiviral treatment of COVID-19. Turk J Med Sci. 2020;50(3):611-9. doi:10.3906/sag-2004-145
13. Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S, et al. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat. 2020;53:100719. doi:10.1016/j.drup.2020.100719
14. Singh H, Chauhan P, Kakkar AK. Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead. Eur J Pharmacol. 2021;890:173717. doi:10.1016/j.ejphar.2020.173717
15. Barzkar F, Ranjbar M, Sioofy-Khojine AB, Khajehazad M, Azad RV, Moradi Y, et al. Efficacy and safety of chloroquine and hydroxychloroquine for COVID-19: A comprehensive evidence synthesis of clinical, animal, and in vitro studies. Med J Islam Repub Iran. 2020;34:171. doi:10.47176/mjiri.34.171
16. Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent Sci. 2020;6(5):672-83. doi:10.1021/acscentsci.0c00489
17. Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med Infect Dis. 2020;35:101647. doi:10.1016/j.tmaid.2020.101647
18. Santoro MG, Carafoli E. Remdesivir: From Ebola to COVID-19. Biochem Ciophys Res Commun. 2021;538:145-50. doi:10.1016/j.bbrc.2020.11.043
19. Uzunova K, Filipova E, Pavlova V, Vekov T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed Pharmacother. 2020;131:110668. doi:10.1016/j.biopha.2020.110668
20. Łagocka R, Dziedziejko V, Kłos P, Pawlik A. Favipiravir in Therapy of Viral Infections. J Clin Med. 2021;10(2):273. doi:10.3390/jcm10020273
21. Saghir SAM, AlGabri NA, Alagawany MM, Attia YA, Alyileili SR, Elnesr SS, et al. Chloroquine and Hydroxychloroquine for the Prevention and Treatment of COVID-19: A Fiction, Hope or Hype? An Updated Review. Ther Clin Risk Manag. 2021;17:371-87. doi:10.2147/tcrm.s301817
22. Zhan X, Dowell S, Shen Y, Lee DL. Chloroquine to fight COVID-19: A consideration of mechanisms and adverse effects? Heliyon. 2020;6(9):e04900. doi:10.1016/j.heliyon.2020.e04900
23. Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289-93. doi:10.1038/s41586-020-2223-y
24. Hafeez A, Naz A, Naeem S, Bano K, Akhtar N. Computational study on the geometry optimization and excited - state properties of riboflavin by ArgusLab 4.0.1. Pak J Pharm Sci. 2013;26(3):487-93.
25. Krishnamoorthy M, Balakrishnan R. Docking studies for screening anticancer compounds of Azadirachta indica using Saccharomyces cerevisiae as model system. J Nat Sci Biol Med. 2014;5(1):108-11. doi:10.4103/0976-9668.127298
26. Kolb P, Irwin JJ. Docking screens: right for the right reasons? Curr Top Med Chem. 2009;9(9):755-70. doi:10.2174/156802609789207091
27. Eweas AF, Alhossary AA, Abdel-Moneim ASA. Molecular Docking Reveals Ivermectin and Remdesivir as Potential Repurposed Drugs Against SARS-CoV-2. Front Microbiol. 2021;11:592908. doi:10.3389/fmicb.2020.592908
28. Mothay D, Ramesh KV. Binding site analysis of potential protease inhibitors of COVID-19 using AutoDock. Virusdisease. 2020;31:194-9. doi:10.1007/s13337-020-00585-z
29. Das S, Sarmah S, Lyndem S, Roy AS. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J Biomol Struct Dyn. 2020:1-11. doi:10.1080/07391102.2020.1763201