In Silico Study for Similar FDA Approved Drugs as Inhibitors of SARS-CoV-2 Spike and the Host Receptor Proteins
Main Article Content
Abstract
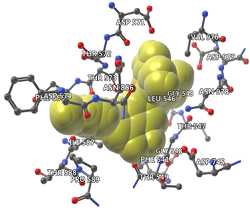
The severe acute respiratory syndrome coronavirus 2, known as COVID-19, has been hideously increased worldwide. The disease began in Wuhan, China, around December 2019, then spread to most countries. Social distancing is the best procedure to prevent infection. Screening the available database containing millions of drug molecules or phytochemicals has become rapid and straightforward because of the computer-aided drug design (CADD) methods. In the present study, 300 phytochemicals and cellulose ether derivatives are screened through a docking study. Docking analysis showed that only four molecules (a-neohesperidin, quercetin 3-O-glucosylrutinoside, 14-ketostypodiol diacetate, and hydroxypropyl methylcellulose) were able to interact with the spike protein. However, two among them (quercetin 3-O-glucosylrutinoside and 14-ketostypodiol diacetate) could interact with the host cell receptor (ACE2) of SARS-CoV-2. The binding affinity of the four compounds is high. Still, according to Lipinski's rule of five, only 14-ketostypodiol diacetate was selected as a drug molecule due to its pharmacokinetic and ADMET properties. Screening for drug analogs to the 14-ketostypodiol diacetate detected five approved drugs. Docking analysis of these drugs with the target proteins showed that the five drugs interact with the host receptor protein, and three interact with viral spike protein. Accordingly, we suggest that molecular docking and drug analogs studies could support rapid drug development. In addition, future perspectives on therapeutic applications of 14-ketostypodiol diacetate are required for using it against SARS-CoV-2 infections.
Downloads
Article Details
Authors continue to retain the copyright to the article if the article is published in the Journal of Molecular Docking. They will also retain the publishing rights to the article without any restrictions.
Authors who publish with this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal, right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles of work (eg, post-institutional repository) or publish it in a book, with acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process, as can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.
References
2. Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56(2):106054. doi:10.1016/j.ijantimicag.2020.106054
3. Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2021;54(2):159-63. doi:10.1016/j.jmii.2020.03.022
4. Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766-88. doi:10.1016/j.apsb.2020.02.008
5. Bosch BJ, van der Zee R, de Haan CAM, Rottier PJM. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801-11. doi:10.1128/jvi.77.16.8801-8811.2003
6. da Silva PG, Mesquita JR, Nascimento MdSJ, Ferreira VAM. Viral, host and environmental factors that favor anthropozoonotic spillover of coronaviruses: An opinionated review, focusing on SARS-CoV, MERS-CoV and SARS-CoV-2. Sci Total Environ. 2021;750:141483. doi:10.1016/j.scitotenv.2020.141483
7. Xia X. Domains and Functions of Spike Protein in Sars-Cov-2 in the Context of Vaccine Design. Viruses. 2021;13(1):109. doi:10.3390/v13010109
8. Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141-9. doi:10.1038/s41401-020-0485-4
9. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281-92. doi:10.1016/j.cell.2020.02.058
10. Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93-116. doi:10.1016/bs.aivir.2019.08.002
11. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444-8. doi:10.1126/science.abb2762
12. Ibrahim IM, Abdelmalek DH, Elshahat ME, Elfiky AA. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80(5):554-62. doi:10.1016/j.jinf.2020.02.026
13. Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent Sci. 2020;6(3):315-31. doi:10.1021/acscentsci.0c00272
14. Ma L, Li H, Lan J, Hao X, Liu H, Wang X, et al. Comprehensive analyses of bioinformatics applications in the fight against COVID-19 pandemic. Comput Biol Chem. 2021;95:107599. doi:10.1016/j.compbiolchem.2021.107599
15. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-3. doi:10.1126/science.abb2507
16. Gui M, Song W, Zhou H, Xu J, Chen S, Xiang Y, et al. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27(1):119-29. doi:10.1038/cr.2016.152
17. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215-20. doi:10.1038/s41586-020-2180-5
18. Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 2020;18(2):152-8. doi:10.1016/j.joim.2020.02.005
19. Depix MS, Martínez J, Santibañez F, Rovirosa J, Martín AS, Maccioni RB. The compound 14-keto-stypodiol diacetate from the algae Stypopodium flabelliforme inhibits microtubules and cell proliferation in DU-145 human prostatic cells. Mol Cell Biochem. 1998;187:191-9. doi:10.1023/A:1006879308861
20. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi:10.1038/srep42717
21. Lionta E, Spyrou G, Vassilatis DK, Cournia Z. Structure-based virtual screening for drug discovery: principles, applications and recent advances. Curr Top Med Chem. 2014;14(16):1923-38. doi:10.2174/1568026614666140929124445
22. Tallei TE, Tumilaar SG, Niode NJ, Fatimawali, Kepel BJ, Idroes R, et al. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (M pro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Scientifica. 2020;2020:6307457. doi:10.1155/2020/6307457
23. Shamkh IM, Pratiwi D. Development of SARS-CoV-2 Inhibitors Using Molecular Docking Study with Different Coronavirus Spike Protein and ACE2. J Mol Docking. 2021;1(1):1-14. doi:10.33084/jmd.v1i1.2212
24. Davies M, Jones RDO, Grime K, Jansson-Löfmark R, Fretland AJ, Winiwarter S, et al. Improving the Accuracy of Predicted Human Pharmacokinetics: Lessons Learned from the AstraZeneca Drug Pipeline Over Two Decades. Trends Pharmacol Sci. 2020;41(6):390-408. doi: https://doi.org/10.1016/j.tips.2020.03.004
25. Mitra AK, Kwatra D, Vadlapudi AD. Drug Delivery. Burlington (MA): Jones and Bartlett Learning; 2015.
26. Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011-33. doi:10.3390/v4061011
27. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80. doi:10.1016/j.cell.2020.02.052
28. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562-9. doi:10.1038/s41564-020-0688-y
29. Desai NC, Kotadiya GM, Trivedi. Studies on molecular properties prediction, antitubercular and antimicrobial activities of novel quinoline based pyrimidine motifs. Bioorg Med Chem Lett. 2014;24(14):3126-30. doi:10.1016/j.bmcl.2014.05.002
30. Ming Y, Qiang L. Involvement of Spike Protein, Furin, and ACE2 in SARS-CoV-2-Related Cardiovascular Complications. SN Compr Clin Med. 2020;Online ahead of print. doi:10.1007/s42399-020-00400-2
31. Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45(12):2615-23. doi:10.1021/jm020017n
32. Purandare AV. Understanding Drug Development: A Primer on the Food and Drug Administration. J Pediatric Infect Dis Soc. 2021;10(10):977-81. doi:10.1093/jpids/piab023