Molecular Docking Studies of Phytoconstituents Identified in Traditional Siddha Polyherbal Formulations Against Possible Targets of SARS-CoV-2
Main Article Content
Abstract
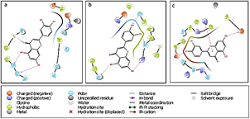
The Indian Traditional Medicines System has long used Siddha polyherbal formulations for different viral diseases. The ingredients of these formulas have been proven to be antiviral. The study focuses on in silico computational evaluation of phytoconstituents of the official Siddha formulation Kabasura, Thonthasura, and Vishasura Kudineer, which were widely used in treating viral fever and respiratory infections and may influence the current SARS-CoV-2 coronary virus pandemic. Maestro interface (Schrödinger Suite, LLC, NY) was used for molecular docking studies against MPro (PDB ID 5R82, 6Y2F, and 6LU7), Nsp15 endoribonuclease (6W01), RNA-dependent RNA polymerase (6M71), and spike protein (6VW1) of SARS-CoV-2. In addition, pharmacokinetics (ADME) and safety profile prediction studies were performed to identify the best drug candidates using Qikpro and Toxicity Estimation Software Tool (T.E.S.T). A total of 36 compounds were screened, of which nine displayed strong binding affinity and drug-likeness. Luteolin and chrysoeriol produced stronger results. These nine compounds were free of oral toxicity as evaluated by the Toxicity estimation software. Based on further in vitro, in vivo, and clinical effectiveness trials, these compounds may be used for the prevention or treatment as per the Indian system of traditional medicines.
Downloads
Article Details
Authors continue to retain the copyright to the article if the article is published in the Journal of Molecular Docking. They will also retain the publishing rights to the article without any restrictions.
Authors who publish with this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal, right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles of work (eg, post-institutional repository) or publish it in a book, with acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process, as can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.
References
2. Kaye AD, Okeagu CN, Pham AD, Silva RA, Hurley JJ, Arron BL, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Pract Res Clin Anaesthesiol. 2020:[Epub ahead of print]. doi:10.1016/j.bpa.2020.11.009
3. Chen B, Liu M, Huang C. Current diagnostic and therapeutic strategies for COVID-19. J Pharm Anal. 2021;11(2):129-37. doi:10.1016/j.jpha.2020.12.001
4. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol. 2020;45(8):100618. doi:10.1016/j.cpcardiol.2020.100618
5. Espinoza D, Rodriguez R, Kowalski A. 24 Hours: A Case of Multiorgan Failure Associated With COVID-19. Cureus. 2020;12(8):e10149. doi:10.7759/cureus.10149
6. Li N, Zhu L, Sun L, Shao G. The effects of novel coronavirus (SARS-CoV-2) infection on cardiovascular diseases and cardiopulmonary injuries. Stem Cell Res. 2021;51:102168. doi:10.1016/j.scr.2021.102168
7. Fernández-Quintela A, Milton-Laskibar I, Trepiana J, Gómez-Zorita S, Kajarabille N, Léniz A, et al. Key Aspects in Nutritional Management of COVID-19 Patients. J Clin Med. 2020;9(8):2589. doi:10.3390/jcm9082589
8. Wadaa-Allah A, Emhamed MS, Sadeq MA, Dahman NBH, Ullah I, Farrag NS, et al. Efficacy of the current investigational drugs for the treatment of COVID-19: a scoping review. Ann Med. 2021;53(1):318-34. doi:10.1080/07853890.2021.1875500
9. Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int J Biol Sci. 2020;16(10):1708-17. doi:10.7150/ijbs.45538
10. Park YL, Canaway R. Integrating Traditional and Complementary Medicine with National Healthcare Systems for Universal Health Coverage in Asia and the Western Pacific. Health Syst Reform. 2019;5(1):24-31. doi:10.1080/23288604.2018.1539058
11. Rudra S, Kalra A, Kumar A, Joe W. Utilization of alternative systems of medicine as health care services in India: Evidence on AYUSH care from NSS 2014. PLoS One. 2017;12(5):e0176916. doi:10.1371/journal.pone.0176916
12. Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J Tradit Complement Med. 2017;7(2):234-44. doi:10.1016/j.jtcme.2016.05.006
13. Jabaris SL, Ananthalakshmi V. The current situation of COVID-19 in India. Brain Behav Immun Health. 2021;100200. doi:10.1016/j.bbih.2021.100200
14. Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021;26(1):80-93. doi:10.1016/j.drudis.2020.10.010
15. Jain J, Pai S, Sunil S. Standardization of in vitro assays to evaluate the activity of polyherbal siddha formulations against Chikungunya virus infection. Virusdisease. 2018;29(1):32-9. doi:10.1007/s13337-018-0421-0
16. Jain J, Kumar A, Narayanan V, Ramaswamy RS, Sathiyarajeswaran P, Devi MSS, et al. Antiviral activity of ethanolic extract of Nilavembu Kudineer against dengue and chikungunya virus through in vitro evaluation. J Ayurveda Integr Med. 2020;11(3):329-35. doi:10.1016/j.jaim.2018.05.006
17. Mahadevan H, Palraj V. Literature Review on Siddha Herbal Formulations (Kudineer) Available for The Management of Dengue. Int J Pharmacol Clin Sci. 2016;5(3):90-6. doi:10.5530/ijpcs.5.3.5
18. Prakash P, Meena R, Stanley A, Swetha S, Govindaraju L, Durgasruthy P, et al. Evidence-based traditional Siddha formulations for prophylaxis and management of respiratory symptoms in COVID-19 pandemic- a review. Biocatal Agric Biotechnol. 2021:[Epub ahead of print]. doi:10.1016/j.bcab.2021.102056
19. Kiran G, Karthik L, Devi MSS, Sathiyarajeswaran P, Kanakavalli K, Kumar KM, et al. In Silico computational screening of Kabasura Kudineer - Official Siddha Formulation and JACOM against SARS-CoV-2 spike protein. J Ayurveda Integr Med. 2020:[Epub ahead of print]. doi:10.1016/j.jaim.2020.05.009
20. Ramalingam V, Venkataramani G. Unlocking the Potential of Traditional Native Medicines - A Perspective to Manage the COVID-19 Pandemic. J Res Trad Med. 2020;6(1):21-8. doi:10.5455/jrtm.2020/95807
21. Rajalakshmi P, Vadivel V, Sriram S, Brindha P. Evaluation of in vitro antioxidant and anti-atherogenic properties of selected Siddha polyherbal decoctions. Int J Res Pharm Sci. 2020;11(2):1707-15. doi:10.26452/ijrps.v11i2.2072
22. Kumar PM, Sundaram KM, Ramasamy MS. Coronavirus spike (S) glycoprotein (2019-ncov) targeted siddha medicines kabasura kudineer and thonthasura kudineer –in silico evidence for corona viral drug. Asian J Pharm Res Health Care. 2020;12(1):20-7. doi:10.18311/ajprhc/2020/25103
23. Shailaja R, Sugunthan S, Kumar MP. A review on polyherbal formulation–Vishasura Kudineer chooranam–A classical anti-viral drug used in Siddha system of medicine. Eur J Pharm Med Res. 2017;4(9):184-92.
24. Douangamath A, Fearon D, Gehrtz P, Krojer T, Lukacik P, Owen CD, et al. Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease. Nat Commun. 2020:11(1):5047. doi:10.1038/s41467-020-18709-w
25. Zhang L, Lin D, Sun X, Curth U, Drosten C, Saurhering L, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409-12. doi:10.1126/science.abb3405
26. Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289-93. doi:10.1038/s41586-020-2223-y
27. Kim Y, Jedrzejczak R, Maltseva NI, Wilamowski M, Endres M, Godzik A, et al. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. Protein Sci. 2020;29(7):1596-605. doi:10.1002/pro.3873
28. Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779-82. doi:10.1126/science.abb7498
29. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221-4. doi:10.1038/s41586-020-2179-y
30. Sinha SK, Shakya A, Prasad SK, Singh S, Gurav NS, Prasad RS, et al. An in-silico evaluation of different Saikosaponins for their potency against SARS-CoV-2 using NSP15 and fusion spike glycoprotein as targets. J Biomol Struct Dyn. 2020:1-12. doi:10.1080/07391102.2020.1762741
31. Shah B, Modi P, Sagar SR. In silico studies on therapeutic agents for COVID-19: Drug repurposing approach. Life Sci. 2020;252:117652. doi:10.1016/j.lfs.2020.117652
32. Ruiz P, Begluitti G, Tincher T, Wheeler J, Mumtaz M. Prediction of Acute Mammalian Toxicity Using QSAR Methods: A Case Study of Sulfur Mustard and Its Breakdown Products. Molecules. 2012;17(8):8982-9001. doi:10.3390/molecules17088982
33. Poli G, Granchi C, Rizzolio F, Tuccinardi T. Application of MM-PBSA Methods in Virtual Screening. Molecules. 2020;25(8):1971. doi:10.3390/molecules25081971
34. Lin J, Sahakian DC, de Morais SMF, Xu JJ, Polzer RJ, Winter SM. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr Top Med Chem. 2003;3(10):1125-54. doi:10.2174/1568026033452096
35. Divyashri G, Murthy TPK, Sundareshan S, Kamath P, Murahari M, Saraswathy GR, et al. In silico approach towards the identification of potential inhibitors from Curcuma amada Roxb against H. pylori: ADMET screening and molecular docking studies. Bioimpacts. 2021;11(2):119-27. doi:10.34172/bi.2021.19
36. Chinedu E, Arome D, Ameh FS. A New Method for Determining Acute Toxicity in Animal Models. Toxicol Int, 2013;20(3):224-6. doi:10.4103/0971-6580.121674
37. Kaleelullah RA, Garugula N. Teratogenic Genesis in Fetal Malformations. Cureus. 2021;13(2):e13149. doi:10.7759/cureus.13149
38. Levy DD, Hakura A, Elespuru RK, Escobar PA, Kato M, Lott J, et al. Demonstrating laboratory proficiency in bacterial mutagenicity assays for regulatory submission. Mutat Res Genet Toxicol Environ Mutagen. 2019;848:403075. doi:10.1016/j.mrgentox.2019.07.005
39. Wang WQ, Duan HX, Pei ZT, Xu RR, Qin ZT, Zhu GC, et al. Evaluation by the Ames Assay of the Mutagenicity of UV Filters Using Benzophenone and Benzophenone-1. Int J Environ Res Public Health. 2018;15(9):1907. doi:10.3390/ijerph15091907
40. Balachandar V, Mahalaxmi I, Kaavya J, Vivekanandhan G, Ajithkumar S, Arul N, et al. COVID-19: emerging protective measures. Eur Rev Med Pharmacol Sci. 2020;24(6):3422-5. doi:10.26355/eurrev_202003_20713
41. Vellingiri B, Jayaramayya K, Iyer M, Narayanasamy A, Govindasamy V, Giridharan B, et al. Sci Total Environ. 2020;725:138277. doi:10.1016/j.scitotenv.2020.138277
42. Mekala P, Krishnamurthy TRG. Phytochemical screening and pharmacological update on Kabasura Kudineer Choornam and Nilavembu Kudineer Choornam P Mekala and TR Gopala Krishna Murthy. J Pharmacogn Phytochem. 2020;9(3):1031-6. doi:10.22271/phyto.2020.v9.i3q.11428
43. Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, Gaurav, et al. Indian Medicinal Plants and Formulations and Their Potential Against COVID-19–Preclinical and Clinical Research. Front Pharmacol. 2020;11:578970. doi:10.3389/fphar.2020.578970
44. Hall Jr DC, Ji HF. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med Infect Dis. 2020;35:101646. doi:10.1016/j.tmaid.2020.101646
45. Ullrich S, Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg Med Chem Lett. 2020;30(17):127377. doi:10.1016/j.bmcl.2020.127377
46. Bian J, Li Z. Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm Sin B. 2021;11(1):1-12. doi:10.1016/j.apsb.2020.10.006
47. Juang Y, Yin W, Xu HE. RNA-dependent RNA polymerase: Structure, mechanism, and drug discovery for COVID-19. Biochem Biophys Res Commun. 2021;538:47-53. doi:10.1016/j.bbrc.2020.08.116
48. Rohaim MA, El Naggar RF, Clayton E, Munir M. Structural and functional insights into non-structural proteins of coronaviruses. Microb Pathog. 2021;150:104641. doi:10.1016/j.micpath.2020.104641