N-acetylation of 2-aminobenzothiazoles with Acetic Acid for Evaluation of Antifungal Activity and In Silico Analysis
Main Article Content
Abstract
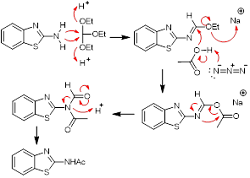
Acetamides (S30A1 and S30) were synthesized from benzo[d]thiazol-2-amine and 6-nitrobenzo[d]thiazol-2-amine by direct use of acetic acid instead of acetylating agents. The usual acetylating agents, acetic anhydride and acetyl chloride are very unstable especially because of their high sensitivity to environmental moisture. Thus, acetylation by direct use of acetic acid was searched as an alternative approach for synthesizing acetanilides. In this study, acetamides were synthesized with a yield of 88% and 82% respectively. The synthesized compounds were then screened for antifungal activity. At a concentration of 300 µg/disc, S30A1 showed 18 mm, 28 mm, 20 mm, and 16 mm zone of inhibitions against Penicillium notatum, Candida albicans, Aspergillus flavus, and Aspergillus niger, respectively. The standard miconazole was used at 50 µg/disc concentration. An in silico analysis was done for the possible binding modes in the C. albicans N-myristoyltransferase enzyme.
Downloads
Article Details
Authors continue to retain the copyright to the article if the article is published in the Journal of Molecular Docking. They will also retain the publishing rights to the article without any restrictions.
Authors who publish with this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal, right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles of work (eg, post-institutional repository) or publish it in a book, with acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process, as can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.
References
2. Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J Fungi. 2017;3(4):57. doi:10.3390/jof3040057
3. Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden Killers: Human Fungal Infections. Sci Transl Med. 2012;4(165):165rv13. doi:10.1126/scitranslmed.3004404
4. Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, et al. Candida auris Isolates Resistant to Three Classes of Antifungal Medications—New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(1):6–9. doi:10.15585/mmwr.mm6901a2
5. Zachary JF. Mechanisms of Microbial Infections. Pathol Basis Vet Dis. 2017;132-241.e1. doi:10.1016/B978-0-323-35775-3.00004-7
6. Cano NH, Ballari MS, López AG, Santiago AN. New Synthesis and Biological Evaluation of Benzothiazole Derivates as Antifungal Agents. J Agric Food Chem. 2015;63(14):3681–6. doi:10.1021/acs.jafc.5b00150
7. Liu Y, Wang Y, Dong G, Zhang Y, Wu S, Miao Z, et al. Novel benzothiazole derivatives with a broad antifungal spectrum: Design, synthesis and structure–activity relationships. MedChemComm. 2013;4(12):1551–61. doi:10.1039/C3MD00215B
8. Pricopie AI, Focșan M, Ionuț I, Marc G, Vlase L, Găină LI, et al. Novel 2,4-Disubstituted-1,3-Thiazole Derivatives: Synthesis, Anti-Candida Activity Evaluation and Interaction with Bovine Serum Albumine. Molecules. 2020;25(5):1079. doi:10.3390/molecules25051079
9. Wang S, Bao L, Song D, Wang J, Cao X, Ke S. Amino acid-oriented poly-substituted heterocyclic tetramic acid derivatives as potential antifungal agents. Eur J Med Chem. 2019;179:567–75. doi:10.1016/j.ejmech.2019.06.078
10. Gjorgjieva M, Tomašič T, Kikelj D, Mašič LP. Benzothiazole-based Compounds in Antibacterial Drug Discovery. Curr Med Chem. 2018;25(38):5218–36. doi:10.2174/0929867324666171009103327
11. Kumari B, Chauhan K, Trivedi J, Jaiswal V, Kanwar SS, Pokharel YR. Benzothiazole-Based-Bioconjugates with Improved Antimicrobial, Anticancer and Antioxidant Potential. ChemistrySelect. 2018;3(40):11326–32. doi:10.1002/slct.201801936
12. Ghosh AK, Brindisi M. Organic Carbamates in Drug Design and Medicinal Chemistry. J Med Chem. 2015;58(7):2895-940. doi:10.1021/jm501371s
13. Piscitelli F, Ballatore C, Smith 3rd AB. Solid phase synthesis of 2-aminobenzothiazoles. Bioorg Med Chem Lett. 2010;20(2):644-8. doi:10.1016/j.bmcl.2009.11.055
14. Morales-Vera R, Crawford J, Dou C, Bura R, Gustafson R. Techno-Economic Analysis of Producing Glacial Acetic Acid from Poplar Biomass via Bioconversion. Molecules. 2020;25(18):4328. doi:10.3390/molecules25184328
15. Sogabe S, Masubuchi M, Sakata K, Fukami TA, Morikami K, Shiratori Y, et al. Crystal Structures of Candida albicans N-Myristoyltransferase with Two Distinct Inhibitors. Chem Biol. 2002;9(10):1119–28. doi:10.1016/S1074-5521(02)00240-5
16. Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–61. doi:10.1002/jcc.21334
17. Mehanna WE, Lu T, Debnath B, Lasheen DS, Serya RAT, Abouzid KA, et al. Synthesis, ADMET Properties, and Biological Evaluation of Benzothiazole Compounds Targeting Chemokine Receptor 2 (CXCR2). ChemMedChem. 2017;12(13):1045–54. doi:10.1002/cmdc.201700229
18. Rothweiler U, Stensen W, Brandsdal BO, Isaksson J, Leeson FA, Engh RA, et al. Probing the ATP-Binding Pocket of Protein Kinase DYRK1A with Benzothiazole Fragment Molecules. J Med Chem. 2016;59(21):9814–24. doi:10.1021/acs.jmedchem.6b01086
19. Orsy G, Fülöp F, Mándity IM. N-Acetylation of Amines in Continuous-Flow with Acetonitrile-No Need for Hazardous and Toxic Carboxylic Acid Derivatives. Molecules. 2020;25(8):1985. doi:10.3390/molecules25081985
20. Tiwari RK, Verma AK, Chhillar AK, Singh D, Singh J, Sankar K, et al. Synthesis and antifungal activity of substituted-10-methyl-1,2,3,4-tetrahydropyrazino[1,2-a]indoles. Bioorg Med Chem. 2006;14(8):2747–52. doi:10.1016/j.bmc.2005.11.054
21. Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021;26(1):80-93. doi:10.1016/j.drudis.2020.10.010
22. Lee H, Lim HK, Kim H. Hydration Thermodynamics of Non-Polar Aromatic Hydrocarbons: Comparison of Implicit and Explicit Solvation Models. Molecules. 2018;23(11):2927. doi:10.3390/molecules23112927
23. Rasulev B. Recent Developments in 3D QSAR and Molecular Docking Studies of Organic and Nanostructures. In: Leszczynski J, Kaczmarek-Kedziera A, Puzyn T, Papadopoulos MG, Reis H, Shukla MK, editors. Handbook of Computational Chemistry. Cham: Springer; 2017. p. 2133-61. doi:10.1007/978-3-319-27282-5_54