Phytochemical Molecules Binding with the Proteins of Mycolic Acid Synthesis Pathway of Mycobacterium tuberculosis
Main Article Content
Abstract
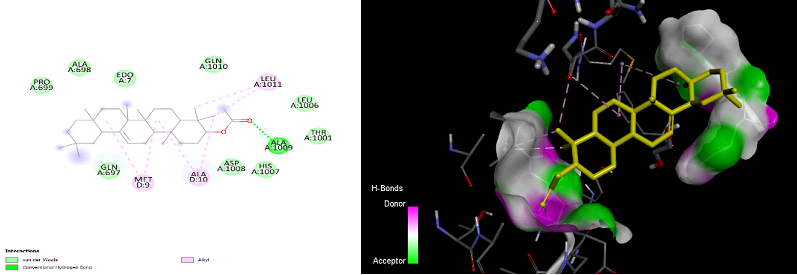
Resistance against anti-tubercular drugs is a significant problem. This elucidates the need for novel drug targets. Altering and targeting the enzymes involved in cell wall synthesis led to fatal damage to the bacterial cell. Mycolic acids are critically responsible for the virulence of Mycobacterium Tuberculosis. This pathway represents an essential reservoir of novel targets for developing new TB drugs. The study aims to identify phytochemicals with the capacity to bind with enzymes of mycolic acid synthesis pathways. This study shows the interaction between phytochemicals and proteins responsible for mycolic acid synthesis is shown through bioinformatics & molecular docking tools. Docking showed binding affinity between protein molecules of the mycolic acid synthesis pathway and ligand molecules in the study. PKS13 (polyketide synthase) interacts with the ligand beta-amyrin acetate with a vina score of -7.1 Kcal/mol. At the same time, its binding energy with Piperine is -6.8 Kcal/mol. DprE1 (Decaprenylphosphoryl-bet-D-ribose-2-epimerase), the other protein docked with beta-amyrin acetate, showed a vina score of -9.7 Kcal/mol binding energy. Piperine with DprE1 exhibits interaction with a score of -8.3 Kcal/mol. Beta-amyrin acetate is docked with a score of -6.9 Kcal/mol against KasA (Beta-ketoacyl-acyl carrier protein synthase). On the other hand, Piperine with KasA gave a result of -7.0 Kcal/mol. Piperine and Beta-amyrin acetate binds to PKS13, DprE1 & KasA protein/enzymes responsible for mycolic acid biosynthesis.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Journal of Molecular Docking. They will also retain the publishing rights to the article without any restrictions.
Authors who publish with this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal, right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles of work (eg, post-institutional repository) or publish it in a book, with acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process, as can lead to productive exchanges, as well as earlier and greater citation of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.
References
To be published