Isolation of Endophytic Fungus from Leaves of Uncaria cordata (Lour.) Merr and Antibacterial Activity Against Propionibacterium acnes and Escherichia coli
Abstract
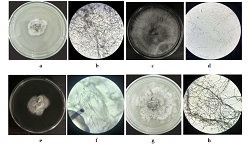
Uncaria cordata (Lour). Merr (akar kaik-kaik) is one of the medicinal plants used as antibacterial because it contains bioactive compounds that can inhibit the growth of microorganisms. The plant is one of the sources of endophyte fungal isolates that can be developed as an alternative to producing antibacterial compounds. This research aimed to isolate the endophytic fungus from the leaves of U. cordata and know the antibacterial activity against Propionibacterium acnes and Escherichia coli by disc diffusion. The Fungi that were isolated from the leaves of U. cordata were 17 isolates. The isolates were continued for antibacterial activity testing: IFED 1 (Nigrospora sp.), IFED 2 (Aspergillus sp.), IFED 3 (Fusarium sp.), and IFED 4, whose genus was unknown. The results obtained were fungal isolates IFED 1 to IFED 4 had activity in inhibiting the growth of P. acnes with moderate category (18.16 mm) and weak categories (6.21, 6.16, and 6.68 mm) and in E. coli with moderate category (14.56 mm) and weak categories (6.53, 6.71, and 7.23 mm). The results of One-Way ANOVA and Tukey's test showed a significant difference (p <0.05) between the diameter of the inhibition zone with the type of endophytic fungus supernatant isolated from the leaves of U. cordata. The best isolate of endophytic fungi inhibiting P. acnes and E. coli bacteria was IFED 1 (Nigrospora sp).
Full text article
References
2. Balloux F, van Dorp L. Q&A: What are pathogens, and what have they done to and for us?BMC Biol.2017;15(1):91. doi:10.1186/s12915-017-0433-z
3. McLaughin J, Watterson S, Layton AM, Bjourson AJ, Barnard E, McDowell A. Propionibacterium acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms. 2019;7(5):128. doi:10.3390/microorganisms7050128
4. Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules. 2020;25(6):1340. doi:10.3390/molecules25061340
5. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277-83.
6. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-41. doi:10.1177/2042098614554919
7. Miozi SH. The role of natural products from medicinal plants against COVID-19: traditional medicine practice in Tanzania. Heliyon. 2022;8(6):e09739. doi:10.1016/j.heliyon.2022.e09739
8. Sewani-Rusike CR, Mammen M. Medicinal plants used as home remedies: a family survey by first year medical students. Afr J Tradit Complement Altern Med. 2014;11(5):67-72. doi:10.4314/ajtcam.v11i5.11
9. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi:10.3389/fphar.2013.00177
10. Sasidharan S, Chen Y, Saravanan D, Sundram KM, Latha LY. Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr J Tradit Complement Altern Med. 2011;8(1):1-10.
11. Abubakar AR, Haque M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J Pharm Bioallied Sci. 2020;12(1):1-10. doi:10.4103/jpbs.jpbs_175_19
12. Gouda S, Das G, Sen SK, Shin HS, Patra JK. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front Microbiol. 2016;7:1538. doi:10.3389/fmicb.2016.01538
13. Mengistu AA. Endophytes: Colonization, Behaviour, and Their Role in Defense Mechanism. Int J Microbiol. 2020;2020:6927219. doi:10.1155/2020/6927219
14. Tiwari P, Bae H. Endophytic Fungi: Key Insights, Emerging Prospects, and Challenges in Natural Product Drug Discovery. Microorganisms. 2022;10(2):360. doi:10.3390/microorganisms10020360
15. Wen J, Okyere SK, Wang S, Wang J, Xie L, Ran Y, et al. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J Fungi. 2022;8(2):205. doi:10.3390/jof8020205
16. Ingle KP, Deshmukh AG, Padole DA, Dudhare MS. Phytochemicals: Extraction Methods, Identification and Detection of Bioactive Compounds from Plant Extracts. J Pharmacogn Phytochem. 2017;6(1):32–6.
17. Ramachandran G, Rajivgandhi G, Maruthupandy M, Manoharan N. Extraction and Partial Purification of Secondary Metabolites from Endophytic Actinomycetes of Marine Green Algae Caulerpa racemosa Against Multi Drug Resistant Uropathogens. Biocatal Agric Biotechnol. 2019;17:750–7. doi:10.1016/j.bcab.2019.01.016
18. Saxena J, Pant V, Sharma MM, Gupta S, Singh A. Hunt for Cellulase Producing Fungi from Soil Samples. J Pure Appl Microbiol. 2015;9(4):2895-902.
19. Gautam CK, Madhav M, Sinha A, Osborne WJ. VIT-CMJ2: Endophyte of Agaricus bisporus in Production of Bioactive Compounds. Iran J Biotechnol. 2016;14(2):19-24. doi:10.15171/ijb.1287
20. Amelia P, Ayunda R, Bahri S. Screening of Antibacterial Activities of the Endophytic Fungi Isolated from the Leaves of Medinilla speciosa Blume. J Fitofarmaka Indones. 2021;8(3):24-8. doi:10.33096/jffi.v8i3.729
21. Barnett HL, Hunter BB. Illustrated Genera of Imperfect Fungi. 4th ed. St. Paul (US): APS Press; 1998.
22. Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species. 3rd ed. Boca Raton (US): CRC Press; 2010.
23. Sciortino Jr CV. Atlas of Clinically Important Fungi. New Jersey (US): John Wiley & Sons; 2017. doi:10.1002/9781119069720
24. Kumala, S. Mikroba Endofit: Pemanfaatan Mikroba Endofit dalam Bidang Farmasi. Jakarta: ISFI Penerbitan; 2014.
25. Diether NE, Willing BP. Microbial Fermentation of Dietary Protein: An Important Factor in Diet⁻Microbe⁻Host Interaction. Microorganisms. 2019;7(1):19. doi:10.3390/microorganisms7010019
26. Rosyida VT, Indrianingsih AW, Maryana R, Wahono SK. Effect of Temperature and Fermentation Time of Crude Cellulase Production by Trichoderma reesei on Straw Substrate. Energy Procedia. 2015;65:368–71. doi:10.1016/j.egypro.2015.01.065
27. Harti AS. Mikrobiologi Kesehatan: Peran Mikrobiologi dalam Kesehatan. Yogyakarta: Andi; 2015.
28. Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2(5):a000414. doi:10.1101/cshperspect.a000414
Authors
Copyright (c) 2022 Melzi Octaviani, Winda Yusma Ameliah, Neni Frimayanti, Meiriza Djohari, Haiyul Fadhli

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.