Design of Experiments Assessment for the Determination of Moisture Content in Five Herbal Raw Materials Contained in Tea Products
Abstract
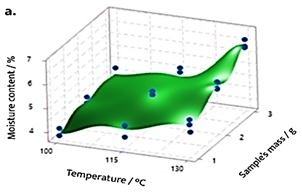
Research interest in natural raw materials is rapidly growing due to the high demand for natural products like herbal teas. Their quality control has a direct impact on safety and efficacy. The aim of this study was to evaluate the impact of sample’s mass and temperature on moisture content in Camellia sinensis (Black tea), Cassia fistula (Senna), Chamaemelum nobile (Chamomille), Lippia alba (Juanilama) and Tilia platyphyllos (Linden) with a gravimetric method developed through a full factorial 32 DoE. A response optimizer was executed in order to establish the test conditions that allow obtaining a response according to a target value from a certified method. DoE’s ANOVA shows reproducibility for Camellia sinensis, Cassia fistula, and Lippia alba. Also, the method’s model is able to explain the response variability for all samples based on the R2 (adj). The composite desirability for the proposed conditions of analysis for the five herbal materials is satisfactory according to each target value. However, the lack of reproducibility in Chamaemelum nobile and Tilia platyphyllos and also, the response prediction problems according to the R2 (pred) for Cassia fistula and Chamaemelum nobile, suggest the execution of further studies for them. Therefore, the present method is considered to be adequate for the analysis of moisture content in Camellia sinensis and Lippia alba raw herbs.
Full text article
References
Asghar, A., Abdullah, Irshad, M.A., & Majeed, M. (2016). Elucidating the therapeutic potential of nutraceuticals. In A. Grumezescu (Ed.), Nutraceuticals (pp. 231-270). Massachusetts, United States: Academic Press. doi: 10.1016/B978-0-12-804305-9.00007-5
Blanco, M.A., Colareda, G.A., van Baren, C., Bandoni, A.L., Ringuelet, J., & Consolini, A.E. (2013). Antispasmodic effects and composition of the essential oils from two South American chemotypes of Lippia alba. Journal of Ethnopharmacology, 149(3), 803–809. doi: 10.1016/j.jep.2013.08.007
Carvalho, P.M.M, Macêdo, C.A.F., Ribeiro, T.F., Silva, A.A., Da Silva, R.E.R., de Morais, L.P., Kerntopf, M.R., Menezes, I.R.A., & Barbosa, R. (2018). Effect of the Lippia alba (Mill.) N.E. Brown essential oil and its main constituents, citral and limonene, on the tracheal smooth muscle of rats. Biotechnology Reports, 17, 31–34. doi: 10.1016/j.btre.2017.12.002
Castillo-Henríquez, L., Vargas-Zúñiga, R., Carazo-Berrocal, G., Madrigal-Redondo, G., Calvo-Guzmán, B., & Baltodano-Viales, E. (2019a). Development of immediate release Rupatadine fumarate 10 mg tablets: A Quality by Design (QbD) approach. Drug Development and Industrial Pharmacy, 45(10), 1674–1681. doi: 10.1080/03639045.2019.1652637
Castillo-Henríquez, L., Madrigal-Redondo, G., Vargas-Zúñiga, R., & Carazo-Berrocal, G. (2019b). Design of Experiments for the Establishment of the Dissolution Test Conditions of Rupatadine Fumarate 10 mg tablets. Journal of Drug Delivery and Therapeutics, 9(1-s), 331–336. doi: 10.22270/jddt.v9i1-s.2359
Chan, E.W.C., Lye, P.Y., Tan, L.N., Eng, S.Y., Tan, Y.P., & Wong, Z.C. (2012). Effects of drying method and particle size on the antioxidant properties of leaves and teas of Morus alba, Lagerstroemia speciosa and Thunbergia laurifolia. Chemical Industry and Chemical Engineering Quarterly, 18(3), 465-472. doi: 10.2298/CICEQ111116023C
Cheng, W.C., Ng, C.S., & Poon, N.L. (2013). Herbal Medicines and Phytopharmaceuticals – Contaminations. In J. Siegel, P. Saukko, & M. Houck (Eds.), Encyclopedia of Forensic Sciences. 2nd edition (pp. 280–288). Amsterdam, Netherlands: Elsevier. doi: 10.1016/B978-0-12-382165-2.00318-4
Conde, R., Corrêa, V.S.C., Carmona, F., Contini, S.H.T., & Pereira, A.M.S. (2011). Chemical composition and therapeutic effects of Lippia alba (Mill.) N. E. Brown leaves hydro-alcoholic extract in patients with migraine. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 18(14), 1197–1201. doi: 10.1016/j.phymed.2011.06.016
Djuris, J., Ibric, S., & Djuric, Z. (2013). Experimental design application and interpretation in pharmaceutical technology. In J. Djuris (Ed.), Computer-Aided Applications in Pharmaceutical Technology (pp. 31–56). Cambridge, England: Woodhead Publishing. doi: 10.1533/9781908818324.31
Fomeni, F.D. (2018). A multi-objective optimization approach for the blending problem in the tea industry. International Journal of Production Economics, 205, 179–192. doi: 10.1016/j.ijpe.2018.08.036
Guimarães, R., Barros, L., Dueñas, M., Calhelha, R.C., Carvalho, A.M., Santos-Buelga, C., Queiroz, M.J.R.P., & Ferreira, I.C.F.R. (2013). Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chemistry, 136(2), 718–725. doi: 10.1016/j.foodchem.2012.08.025
Gutiérrez-Pulido, H. & De la Vara-Salazar, R. (2008). Análisis y diseño de experimentos Segunda edición. Santa Fe, México: McGrawHill Interamericana.
Harbourne, N., Jacquier, J.C., & O’Riordan, D. (2009). Optimization of the extraction and processing conditions of chamomile (Matricaria chamomilla L.) for incorporation into a beverage. Food Chemistry, 115(1), 15–19. doi: 10.1016/j.foodchem.2008.11.044
Kaur, L., Jayasekera, S., & Moughan, P.J. (2014). Antioxidant Quality of Tea (Camellia sinensis) as Affected by Environmental Factors. In V. Preedy (Ed.), Processing and Impact on Antioxidants in Beverages (pp. 121–129). Massachusetts, United States: Academic Press. doi: 10.1016/B978-0-12-404738-9.00013-1
Krempski-Smejda, M., Stawczyk, J., Śmigielski, K., & Prusinowska, R. (2015). Drying of Herbal Product in Closed System. Drying Technology, 33(13), 1671–1677. doi: 10.1080/07373937.2015.1066384
Kumar, A.K., Satish, S., Sayeed, I., & Hedge, K. (2017). Therapeutic Uses of Cassia Fistula: Review. International Journal of Pharma and Chemical Research, 3(1), 38-43.
Li, S., Li, S.K., Li, H.B., Xu, X.R., Deng, G.F., & Xu, D.P. (2014). Antioxidant Capacities of Herbal Infusions. In V. Preedy (Ed.), Processing and Impact on Antioxidants in Beverages (pp. 41–50). Massachusetts, United States: Academic Press. doi: 10.1016/B978-0-12-404738-9.00005-2
Lin, X., Chen, Z., Zhang, Y., Luo, W., Tang, H., Deng, B., & Li, B. (2015). Comparative characterization of green tea and black tea cream: Physicochemical and phytochemical nature. Food Chemistry, 173, 432–440. doi: 10.1016/j.foodchem.2014.10.048
Lin, J., Lu, Z., Yang, H., & Wang, P. (2011). A design of experiments assessment of moisture content in uncured adhesive on static strength of adhesive-bonded galvanized SAE1006 steel. International Journal of Adhesion and Adhesives, 31(6), 478–485. doi: 10.1016/j.ijadhadh.2011.04.001
Mandal, M., Misra, D., Ghosh, N.N., & Mandal, V. (2017). Physicochemical and elemental studies of Hydrocotyle javanica Thunb. For standardization as herbal drug. Asian Pacific Journal of Tropical Biomedicine, 7(11), 979–986. doi: 10.1016/j.apjtb.2017.10.001
Martínez, A.L., González-Trujano, M.E., Aguirre-Hernándeza, E., Moreno, J., Soto-Hernández, M., & López-Muñoz, F.J. (2009). Antinociceptive activity of Tilia americana var. Mexicana inflorescences and quercetin in the formalin test and in an arthritic pain model in rats. Neuropharmacology, 56(2), 564–571. doi: 10.1016/j.neuropharm.2008.10.010
Mead, R., Gilmour, S.G., & Mead, A. (2012). Statistical Principles for the Design of Experiments: Applications to Real Experiments. Cambridge, United Kingdom: Cambridge University Press. doi: 10.1017/CBO9781139020879
Megías-Pérez, R., Shevchuk, A., Zemedie, Y., & Kuhnert, N. (2019). Characterization of commercial green tea leaves by the analysis of low molecular weight carbohydrates and other quality indicators. Food Chemistry, 290, 159-167. doi: 10.1016/j.foodchem.2019.03.069
Mizukami, Y., Sawai, Y., & Yamaguchi, Y. (2006). Moisture Content Measurement of Tea Leaves by Electrical Impedance and Capacitance. Biosystems Engineering, 93(3), 293–299. doi: 10.1016/j.biosystemseng.2005.12.009
Mora-Román, J.J., Agüero-Brenes, N., Angulo-Morales, C., Castro-Solís, J., Hidalgo-Carrillo, G., van Hoof-Gómez, M., Loría-Gutiérrez, A., & Blanco-Barrantes, J. (2018a). Physicochemical and Microbiological Assays for Quality Evaluation of a Brand of Mentha piperita Tisanes in Costa Rica Market: Employment of the Central American Technical Regulation. Journal of Drug Delivery and Therapeutics, 8(5), 329-337. doi: 10.22270/jddt.v8i5.1878
Mora-Román, J.J., Alvarado-Fernández, M. J., Apú-Leitón, N., Arroyo-Solórzano, J. D., Espeleta-González, D., Piedra-Navarro, H., & Blanco-Barrantes, J. (2018b). Pruebas fisicoquímicas para la evaluación de la calidad de una marca costarricense de tisanas de valeriana. Revista Médica de la Universidad de Costa Rica, 12(1), 15-26.
Mukherjee, P.K. (2019a). Quality Evaluation of Herbal Medicines: Challenges and Opportunities. In P.K. Mukherjee (Ed.), Quality Control and Evaluation of Herbal Drugs (pp. 53–77). Amsterdam, Netherlands: Elsevier. doi: 10.1016/B978-0-12-813374-3.00003-X
Mukherjee, P.K. (2019b). Qualitative Analysis for Evaluation of Herbal Drugs. In P.K. Mukherjee (Ed.), Quality Control and Evaluation of Herbal Drugs (pp. 79–149). Amsterdam, Netherlands: Elsevier. doi: 10.1016/B978-0-12-813374-3.00004-1
Mukherjee, P.K. (2019c). Safety-Related Quality Issues for the Development of Herbal Drugs. In P.K. Mukherjee (Ed.), Quality Control and Evaluation of Herbal Drugs (pp. 655–683). Amsterdam, Netherlands: Elsevier. doi: 10.1016/B978-0-12-813374-3.00018-1
Mukkula, A.R.G. & Paulen, R. (2019). Optimal experiment design in nonlinear parameter estimation with exact confidence regions. Journal of Process Control, 83, 187–195. doi: 10.1016/j.jprocont.2019.01.004
Nunes, M.R., Castilho, M.D.S.M., Veeck, A.P.D.L., da Rosa, C.G., Noronha, C.M., Maciel, M.V.O.B, & Barreto, P.M. (2018). Antioxidant and antimicrobial methylcellulose films containing Lippia alba extract and silver nanoparticles. Carbohydrate Polymers, 192, 37–43. doi: 10.1016/j.carbpol.2018.03.014
Orphanides, A., Goulas, V., & Gekas, V. (2013). Effect of drying method on the phenolic content and antioxidant capacity of spearmint. Czech Journal of Food Sciences, 31(5), 509–513. doi: 10.17221/526/2012-CJFS
Reichert, I., Olney, P., & Lahmer, T. (2019). Influence of the error description on model-based design of experiments. Engineering Structures, 193, 100–109. doi: 10.1016/j.engstruct.2019.05.002
Rodino, S. & Butu, M. (2019). Herbal Extracts—New Trends in Functional and Medicinal Beverages. In A. Grumezescu & A. Holban (Eds.), Functional and Medicinal Beverages (pp. 73–108). Massachusetts, United States: Academic Press. doi: 10.1016/B978-0-12-816397-9.00003-0
Sahoo, N., Manchikanti, P., & Dey, S. (2010). Herbal drugs: Standards and regulation. Fitoterapia, 81(6), 462–471. doi: 10.1016/j.fitote.2010.02.001
Schinabeck, T.M., Weigler, F., Mellmann, J., Idler, C., & Flöter, E. (2019). Dynamics of the particle moisture distribution during storage of wheat under laboratory and pilot-scale conditions. Journal of Stored Products Research, 82, 54–66. doi: 10.1016/j.jspr.2019.04.001
Singh, V.K., Das, S., Dwivedy, A.K., Rathore, R., & Dubey, N.K. (2019). Assessment of chemically characterized nanoencapuslated Ocimum sanctum essential oil against aflatoxigenic fungi contaminating herbal raw materials and its novel mode of action as methyglyoxal inhibitor. Postharvest Biology and Technology, 153, 87–95. doi: 10.1016/j.postharvbio.2019.03.022
Steinhoff, B. (2019). Review: Quality of herbal medicinal products: State of the art of purity assessment. Phytomedicine, 60, 153003. doi: 10.1016/j.phymed.2019.153003
Teles, S., Pereira, J.A., Santos, C.H.B., Menezes, R.V., Malheiro, R., Lucchese, A.M., & Silva, F. (2012). Geographical origin and drying methodology may affect the essential oil of Lippia alba (Mill) N.E. Brown. Industrial Crops and Products, 37(1), 247–252. doi: 10.1016/j.indcrop.2011.12.029
Thomas, B.F. & ElSohly, M.A. (2016). Analytical Methods in Formulation Development and Manufacturing. In B.F. Thomas & M. ElSohly (Eds.), The Analytical Chemistry of Cannabis (pp. 63–81). Amsterdam, Netherlands: Elsevier. doi: 10.1016/B978-0-12-804646-3.00004-7
Toontom, N., Meenune, M., Posri, W., & Lertsiri, S. (2012). Effect of drying method on physical and chemical quality, hotness and volatile flavour characteristics of dried chilli. International Food Research Journal, 19(3), 1023-1031.
Vargas, R. & Vecchietti, A. (2016). Influence of raw material moisture on the synthesis of black tea production process. Journal of Food Engineering, 173, 76–84. doi: 10.1016/j.jfoodeng.2015.10.043
Wagner Jr., J.R., Mount III, E.M., & Giles Jr., H.F. (2014). Design of Experiments. In J.R. Wagner Jr., E.M. Mount III, & H.F. Giles Jr. (Eds.), Extrusion 2nd Edition (pp. 291-308). New York, United States: William Andrew. doi: 10.1016/B978-1-4377-3481-2.00025-9
World Health Organization. (1998). Quality control methods for medicinal plant materials. Retrieved from https://apps.who.int/iris/handle/10665/41986
Yang, Y. & Deng, J. (2016). Analysis of pharmaceutical products and herbal medicines using ambient mass spectrometry. TrAC Trends in Analytical Chemistry, 82, 68-88. doi: 10.1016/j.trac.2016.04.011
Zambrano, M.V., Dutta, B., Mercer, D.G., MacLean, H.L., & Touchie, M.F. (2019). Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends in Food Science & Technology, 88, 484–496. doi: 10.1016/j.tifs.2019.04.006
Authors
Copyright (c) 2020 Luis Castillo, Eleaneth Baltodano, Nils Ramírez, Rolando Vargas, Georgia Hanley

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.