Column Chromatography Fractionation and Antioxidant Activity of Passiflora foetida Leaves
Abstract
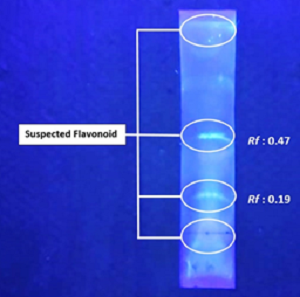
Available synthetic antioxidants have been reported to have mutagenic and toxic effects. On the other hand, natural antioxidants show their superiority as they are not or less toxic. Passiflora foetida has the potential as an antioxidant, but the investigation of the antioxidant activity of the P. foetida chromatography column fraction has not been reported. This studied aims to investigate the antioxidant activity of the column chromatographic fractions of P. foetida leaves. An antioxidant assay using the DPPH and FRAP methods. The extraction was carried out by graded maceration, then fractionation using column chromatography. The antioxidant activity test was carried out using the DPPH and FRAP methods. Thin Layer Chromatography analysis was performed to determine the chromatogram pattern. The EC50 using DPPH method from n-hexane extract: 129.035 µg/mL, ethyl acetate extract: 206.398 µg/mL, methanol extract: 97.453 µg/mL, while the EC50 using FRAP method from n-hexane extract: 67.851 µg/mL, ethyl acetate extract : 68.981 µg/mL, and methanol extract: 58.787 µg/mL. Column chromatography fractions have antioxidant activity, with FMetPF6 as the fraction with the best activity, with percent inhibition 41.85±1.96 at concentration 25 µg/mL (DPPH), and with percent antioxidant activity 26.03±0.84 at concentration 9 µg/mL (FRAP). Passiflora foetida leaves have great potential as an antioxidant; both the extract and its fractions have antioxidant activity. The FMetPF6 has the best activity compare to other extracts and fractions. Further analysis to determine the various compounds in FMetPF6 using LC-MS/MS will facilitate the active compound's isolation.
Full text article
References
2. Martemucci G, Costagliola C, Mariano M, D’andrea L, Napolitano P, D’Alessandro AG. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen. 2022;2(2):48-78. doi:10.3390/oxygen2020006
3. Sharifi-Rad M, Kumar NVA, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. 2020;11:694. doi:10.3389/fphys.2020.00694
4. Patel J, Baptiste BA, Kim E, Hussain M, Croteau DL, Bohr VA. DNA damage and mitochondria in cancer and aging. Carcinogenesis. 2020;41(12):1625-34. doi:10.1093/carcin/bgaa114
5. Flieger J, Flieger W, Baj J, Maciejewski R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials. 2021;14(15):4135. doi:10.3390/ma14154135
6. Lourenço SC, Moldão-Martins M, Alves VD. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules. 2019;24(22):4132. doi:10.3390/molecules24224132
7. Zehiroglu C, Sarikaya SBO. The importance of antioxidants and place in today's scientific and technological studies. J Food Sci Technol. 2019;56(11):4757-74. doi:10.1007/s13197-019-03952-x
8. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018;5(3):93. doi:10.3390/medicines5030093
9. Candra KP, Sulika, Rachmawati M, Rahmadi A, Rohmah M, Ramdan IM, et al. The Effect of Passiflora foetida L. Leaves Decoction on Blood Pressure Profile and Its Correlation with the Demographics of Hypertensive Patients. Trad Integr Med. 2023;8(1):32-9. doi:10.18502/tim.v8i1.12401
10. Siriwardhene MA, Abeysekera AM, Chandrika UG, Goonetilleke AKE. Antihyperglycemic effect and phytochemical screening of aqueous extract of Passiflora foetida (Linn.) on normal Wistar rat model. Afr J Pharm Pharmacol. 2013;7(45):2892-4. doi:10.5897/AJPP10.055
11. Asadujjaman M, Mishuk AU, Hossain MA, Karmakar UK. Medicinal potential of Passiflora foetida L. plant extracts: biological and pharmacological activities. J Integr Med. 2014;12(2):121–6. doi:10.1016/S2095-4964(14)60017-0
12. Patil AS, Paikrao HM. Bioassay Guided Phytometabolites Extraction for Screening of Potent Antimicrobials in Passiflora foetida L. J Appl Pharm Sci. 2012;2(9):137–42. doi:10.7324/JAPS.2012.2927
13. Khaerati K, Ihwan, Maya MS. Antidiabetic Activity Test of Rambusa (Passiflora foetida L) Leaves Extract on Mice (Mus musculus) Induced By Glucose. J Farmasi Galenika Galenika J Pharm. 2015;1(2):99–104. doi:10.22487/j24428744.2015.v1.i2.6240
14. Mulyani E. Studi In Vitro: Efek Anti Kolesterol Ekstrak Daun Rambusa (Passiflora foetida L): In Vitro Study: Anti-Cholesterol Effect of Rambusa Leaf Extract (Passiflora foetida L). J Surya Medika. 2019;4(2):60-5. doi:10.33084/jsm.v4i2.606
15. Trinovita E, Fatmaria. Evaluation of Gastroprotective Activity of Cemot Leaves (Passiflora foetida L.) Extracted by Ultrasonic Assisted Extraction (UAE) Against Ethanol-Induced Gastric Lesions in Rats. Majalah Obat Tradisional. 2020;25(2):108–15. doi:10.22146/mot.53691
16. Shankar D, Basker S, Karthik S. In Vitro Cytotoxic and Apoptotic Effect of Passiflora foetida Against Cervical Cancer Cells and Its Fourier Transform Infrared Profiling. Asian J Pharm Clin Res. 2017;10(11):46–9. doi:10.22159/ajpcr.2017.v10i11.20226
17. Asir PJ, Priyanga S, Hemmalakshmi S, Devaki K. In Vitro Free Radical Scavenging Activity and Secondary Metabolites in Passiflora foetida L. Asian J Pharm Res Health Care. 2012;6(2):3–11.
18. Filho AAM, Kamezaki ÂK, Ribeiro PRE, De Melo ACGR, Fernández IM, Dos Santos RC, et al. Chemical Composition, Antioxidant and Biological Activity of Leaves Passiflora foetida. Chem Eng. 2018;64:241–6. doi:10.3303/CET1864041
19. Song Y, Wei X, Li M, Duan X, Sun Y, Yang R, et al. Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida. Molecules. 2018;23(459):459. doi:10.3390/molecules23020459
20. Ajane GA, Patil AS. Evaluation of Antioxidant Potential of Passiflora foetida Extract and Quantitative Evaluation of its Phytochemical Content- A Possible Natural Antioxidant. Pharm Chem J. 2019;6(4):14–24.
21. Mulia DS, Mulyani E, Rizki MI, Pratama MRF. Rambusa (Passiflora foetida L) vs. Free Radicals: In Vitro Study with DPPH Method. J Pharmascience. 2019;6(2):1–7. doi:10.20527/jps.v6i2.7344
22. Tandoro Y, Widyawati PS, Budianta TDW, Sumargo G. Phytochemical Identification and Antioxidant Activity of Passiflora Foetida Fruits and Leaves Extracts: A Comparative Study. Int J Pharm Pharm Sci. 2020;12(6):55–8. doi:10.22159/ijpps.2020v12i6.31505
23. Sisin NNT, Abdullah H, Sul’ain MD. Antiproliferative, Antioxidative and Compounds Identification from Methanolic Extract of Passiflora Foetida and Its Fractions. J Anal Pharm Res. 2017;6(1)00166. doi:10.15406/japlr.2017.06.00166
24. Schellinger AP, Stoll DR, Carr PW. High speed gradient elution reversed phase liquid chromatography of bases in buffered eluents. Part II. Full equilibrium. J Chromatogr A. 2008;1192(1):54-61. doi:10.1016/j.chroma.2008.02.049
25. Pobłocka-Olech L, Migas P, Krauze-Baranowska M. TLC determination of some flavanones in the buds of different genus Populus species and hybrids. Acta Pharm. 2018;68(2):199–210. doi:10.2478/acph-2018-0018
26. Puspitaningrum A, Elya B, Katrin. Isolation and Characterization of Antioxidant Compounds from Ethyl Acetate and Methanol Extracts of Garcinia daedalanthera Pierre Leaves. Int J App Pharm. 2018;10(1):276-8. doi:10.22159/ijap.2018.v10s1.61
27. Simão MJ, Barboza TJS, Vianna MG, Garcia R, Mansur E, Ignacio ACPR, et al. A comparative study of phytoconstituents and antibacterial activity of in vitro derived materials of four Passiflora species. An Acad Bras Cienc. 2018;90(3):2805–13. doi:10.1590/0001-3765201820170809
28. Birudu RB, Naik MJ, Revana DS, Jilani SK, Janardhan M. Phytochemical Screening of Ethanolic Extract of Passiflora foetida (Linn) and Medicinal Importance. Indian J Res Pharm Biotechnol. 2015;3(4):324–7.
29. Kairupan CF, Mantiri FR, Rumende RRH. Phytochemical Screening and Antioxidant Activity of Ethanol Extract of Leilem (Clerodendrum minahassae Teijsm. & Binn) as an Antihyperlipidemic and Antiatherosclerotic Agent. IOP Conf Ser Earth Environ Sci. 2019;217:012016. doi:10.1088/1755-1315/217/1/012016
30. Gonbad RA, Afzan A, Karimi E, Sinniah UR, Swamy MK. Phytoconstituents and antioxidant properties among commercial tea (Camellia sinensis L.) clones of Iran. Electron J Biotechnol. 2015;18(6):433–8. doi:10.1016/j.ejbt.2015.08.007
31. Mahayasih PGMW, Elya B, Hanafi, M. Fractionation and antioxidant activity potency of the extract of Garcinia lateriflora Blume var. javanica Boerl leaf. AIP Conf Proceed. 2018;1933:030018. doi:10.1063/1.5023965
32. Martha S, Elya B, Hanafi, M. Comparison of inhibitory activity against the α-glucosidase enzymes in the extracts and fractions from leaves of the Garcinia kydia Roxburgh. Asian J Pharm Clin Res. 2017;10(7):401–4. doi:10.22159/ajpcr.2017.v10i7.18529
33. Triadisti N, Sauriasari R, Elya B. Fractionation and α-glucosidase inhibitory activity of fractions from garcinia hombroniana pierre leaves extracts. Pharmacogn J. 2017;9(4):488–92. doi:10.5530/pj.2017.4.79
34. Seelinger M, Popescu R, Seephonkai P, Singhuber J, Giessrigl B, Unger C, et al. Fractionation of an extract of Pluchea odorata separates a property indicative for the induction of cell plasticity from one that inhibits a neoplastic phenotype. Evid Based Complement Alternat Med. 2012;2012:701927. doi:10.1155/2012/701927
35. Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of fl avonoids. Fitoterapia. 2011;82(524):513–23. doi:10.1016/j.fitote.2011.01.018
36. Suratno, Rizki MI, Pratama MRF. In-Vitro Study of Antioxidant Activities from Ethanol Extracts of Akar Kuning (Arcangelisia flava). J Surya Medika. 2019;4(2):66-71. doi:10.33084/jsm.v4i2.594
37. Vo QV, Nam PC, Thong NM, Trung NT, Phan CTD, Mechler A. Antioxidant Motifs in Flavonoids: O-H versus C-H Bond Dissociation. ACS Omega. 2019;4(5):8935-42. doi:10.1021/acsomega.9b00677
38. Ciumărnean L, Milaciu MV, Runcan O, Vesa SC, Răchișan AL, Negrean V, et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules. 2020;25(18):4320. doi:10.3390/molecules25184320
39. Pereira AB, da Silva AM, Barroca MJ, Marques PMM, Braga SS. Physicochemical properties , antioxidant action and practical application in fresh cheese of the solid inclusion compound y-cyclodextrin.quercetin, in comparison with b -cyclodextrin.quercetin. Arab J Chem. 2020;13(1):205–15. doi:10.1016/j.arabjc.2017.04.001
40. Ozgen S, Kilinc OK, Selamoglu Z. Antioxidant Activity of Quercetin : A Mechanistic Review. Turkish J Agric Food Sci Technol. 2016;4(12):1134–8. doi:10.24925/turjaf.v4i12.1134-1138.1069
41. Shamsudin NF, Ahmed QU, Mahmood S, Shah SAA, Sarian MN, Khattak MMAK, et al. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int J Mol Sci. 2022;23(20):12605. doi:10.3390/ijms232012605
Authors
Copyright (c) 2023 Nita Triadisti, Irfan Zamzani

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.