An Overview on Patient-Centered Clinical Services
Abstract
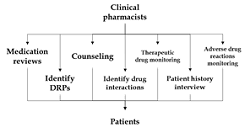
Drug-related problems (DRPs) had often been a concern in the system that needed to be detected, avoided, and addressed as soon as possible. The need for a clinical pharmacist becomes even more important. He is the one who can not only share the load but also be an important part of the system by providing required advice. They fill out the patient's pharmacotherapy reporting form and notify the medical team's head off any drug-related issues. General practitioners register severe adverse drug reactions (ADRs) yearly. As a result of all of this, a clinical pharmacist working in and around the healthcare system is expected to advance the pharmacy industry. Its therapy and drugs can improve one's health quality of life by curing, preventing, or diagnosing a disease, sign, or symptom. The sideshows, on the other hand, do much harm. Because of the services they offer, clinical pharmacy has grown in popularity. To determine the overall effect and benefits of the emergency department (ED) clinical pharmacist, a systematic review of clinical practice and patient outcomes will be needed. A clinical pharmacist's anatomy, toxicology, pharmacology, and medicinal chemistry expertise significantly improves a patient's therapy enforcement. It is now important to examine the failure points of healthcare systems as well as the individuals involved.
Full text article
References
2. Tegegn HG, Abdela OA, Mekuria AB, Bhagavathula AS, Ayele AA. Challenges and opportunities of clinical pharmacy services in Ethiopia: A qualitative study from healthcare practitioners’ perspective. Pharm Pract. 2018;16(1):1121. doi:10.18549/PharmPract.2018.01.1121
3. Visacri MB, Figueiredo IV, Lima TdM. Role of pharmacist during the COVID-19 pandemic: A scoping review. Res Social Adm Pharm. 2021;17(1):1799-806. doi:10.1016/j.sapharm.2020.07.003
4. Francis J, Abraham S. Clinical pharmacists: Bridging the gap between patients and physicians. Saudi Pharm J. 2014;22(6):600-2. doi:10.1016/j.jsps.2014.02.011
5. Tahniyath F. Clinical Pharmacist- A Need for the Society. Indian J Pharm Pract. 2017;10(1):59-61. doi:10.5530/ijopp.10.1.12
6. Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S, et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6(11):e1196-e1252. doi:10.1016/s2214-109x(18)30386-3
7. Thimbleby H. Technology and the Future of Healthcare. J Public Health Res. 2013;2(3):e28. doi:10.4081/jphr.2013.e28
8. Lim XY, Yeo QQ, Kng GLL, Chung WL, Yap KZ. Validation of a Drug-Related Problem Classification System for the Intermediate and Long-Term Care Setting in Singapore. Pharmacy. 2018;6(4):109. doi:10.3390/pharmacy6040109
9. Cipolle R, Strand LM, Morley PC. Pharmaceutical Care Practice. the clinician’s Guide, 3rd Edition. New York, NY: McGraw Hill; 2012.
10. Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30(3):205-11. doi:10.1093/ageing/30.3.205
11. Shiovitz TM, Bain EE, McCann DJ, Skolnick P, Laughren T, Hanina A, et al. Mitigating the Effects of Nonadherence in Clinical Trials. J Clin Pharmacol. 2016;56(9):1151-64. doi:10.1002/jcph.689
12. Ofori-Asenso R, Agyeman AA. Irrational Use of Medicines—A Summary of Key Concepts. Pharmacy. 2016;4(4):35. doi:10.3390/pharmacy4040035
13. Denis JL, van Gestel N. Medical doctors in healthcare leadership: theoretical and practical challenges. BMC Health Serv Res. 2016;16(Suppl 2):158. doi:10.1186/s12913-016-1392-8
14. Salmond SW, Echevarria M. Healthcare Transformation and Changing Roles for Nursing. Orthop Nurs. 2017;36(1):12-25. doi:10.1097/NOR.0000000000000308
15. Niriayo YL, Kumela K, Kassa TD, Angamo MT. Drug therapy problems and contributing factors in the management of heart failure patients in Jimma University Specialized Hospital, Southwest Ethiopia. PLoS One. 2018;13(10):e0206120. doi:10.1371/journal.pone.0206120
16. Khalil H, Huang C. Adverse drug reactions in primary care: a scoping review. BMC Health Serv Res. 2020;20(1):5. doi:10.1186/s12913-019-4651-7
17. Movva R, Jampani A, Nathani J, Pinnamaneni SH, Challa SR. A prospective study of incidence of medication-related problems in general medicine ward of a tertiary care hospital. J Adv Pharm Technol Res. 2015;6(4):190-4. doi:10.4103/2231-4040.166502
18. Hua XL, Gu M, Zeng F, Hu H, Zhou T, Zhang Y, et al. Pharmacy administration and pharmaceutical care practice in a module hospital during the COVID-19 epidemic. J Am Pharm Assoc. 2020;60(3):431-8.ei. doi:10.1016/j.japh.2020.04.006
19. Chalasani SH, Ramesh M, Gurumurthy P. Pharmacist-Initiated Medication Error-Reporting and Monitoring Programme in a Developing Country Scenario. Pharmacy. 2018;6(4):133. doi:10.3390/pharmacy6040133
20. Noormandi A, Karimzadeh I, Mirjalili M, Khalili H. Clinical and economic impacts of clinical pharmacists’ interventions in Iran: a systematic review. DARU J Pharm Sci. 2019;27:361-78. doi:10.1007/s40199-019-00245-8
21. Jafarian K, Allameh Z, Memarzadeh M, Saffaei A, Peymani P, Sabzghabaee AM. The Responsibility of Clinical Pharmacists for the Safety of Medication Use in Hospitalized Children: A Middle Eastern Experience. J Res Pharm Pract. 2019;8(2):83-91. doi:10.4103/jrpp.JRPP_19_66
22. Taberna M, Moncayo FG, Jané-Salas E, Antonio M, Arribas L, Vilajosana E, et al. The Multidisciplinary Team (MDT) Approach and Quality of Care. Front Oncol. 2020;10:85. doi:10.3389/fonc.2020.00085
23. Haines A, Perkins E, Evans EA, McCabe R. Multidisciplinary team functioning and decision making within forensic mental health. Ment Health Rev. 2018;23(3):185-96. doi:10.1108/MHRJ-01-2018-0001
24. Shipkova M, Christians U. Improving therapeutic decisions: Pharmacodynamic monitoring as an integral part of Therapeutic Drug Monitoring. Ther Drug Monit. 2019;41(2):111-4. doi:10.1097/FTD.0000000000000627
25. Buclin T, Thoma Y, Widmer N, André P, Guidi M, Csajka C, et al. The Steps to Therapeutic Drug Monitoring: A Structured Approach Illustrated With Imatinib. Front Pharmacol. 2020;11:177. doi:10.3389/fphar.2020.00177
26. Kang JS, Lee MH. Overview of Therapeutic Drug Monitoring. Korean J Intern Med. 2009;24(1):1-10. doi:10.3904/kjim.2009.24.1.1
27. Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med. 2016;16(5):481-5. doi:10.7861/clinmedicine.16-5-481
28. Shamim S, Sharib SM, Malhi SM, Muntaha S, Raza H, Ata S, et al. Adverse drug reactions (ADRS) reporting: awareness and reasons of under-reporting among health care professionals, a challenge for pharmacists. Springerplus. 2016;5(1):1778. doi:10.1186/s40064-016-3337-4
29. Wasserfallen JB, Livio F, Buclin T, Tillet L, Yersin B, Biollaz J. Rate, type, and cost of adverse drug reactions in emergency department admissions. Eur J Intern Med. 2001;12(5):442-7. doi:10.1016/s0953-6205(01)00159-5
30. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200-5. doi:10.1001/jama.279.15.1200
31. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15-9. doi:10.1136/bmj.329.7456.15
32. Courtman BJ, Stalling SB. Characterization of drug-related problems in elderly patients on admission to a medical ward. Can J Hosp Pharm. 1995;48(3):161-6.
33. Sanii Y, Torkamandi H, Gholami K, Hadavand N, Javadi M. Role of pharmacist counseling in pharmacotherapy quality improvement. J Res Pharm Pract. 2016;5(2):132-7. doi:10.4103/2279-042X.179580
34. Hepler CD. Clinical pharmacy, pharmaceutical care, and the quality of drug therapy. Pharmacotherapy. 2004;24(11):1491-8. doi:10.1592/phco.24.16.1491.50950
35. Elden NMK, Ismail A. The Importance of Medication Errors Reporting in Improving the Quality of Clinical Care Services. Glob J Health Sci. 2016;8(8):243-251. doi:10.5539/gjhs.v8n8p243
36. Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol. 2008;102(3):275-80. doi:10.1111/j.1742-7843.2007.00206.x
37. Tasaka Y, Tanaka A, Yasunaga D, Asakawa T, Araki H, Tanaka M. Potential drug-related problems detected by routine pharmaceutical interventions: safety and economic contributions made by hospital pharmacists in Japan. J Pharm Health Care Sci. 2018;4:33. doi:10.1186/s40780-018-0125-z
38. Bragazzi NL, Mansour M, Bonsignore A, Ciliberti R. The Role of Hospital and Community Pharmacists in the Management of COVID-19: Towards an Expanded Definition of the Roles, Responsibilities, and Duties of the Pharmacist. Pharmacy. 2020;8(3):140. doi:10.3390/pharmacy8030140
39. Alsairafi Z, Waheedi M, Alsaleh F. The perspectives of patients and physicians on the role of pharmacists in improving medication adherence in type 2 diabetes: a qualitative study. Patient Prefer Adherence. 2019;13:1527-43. doi:10.2147/PPA.S218068
40. Vinterflod C, Gustafsson M, Mattsson S, Gallego G. Physicians’ perspectives on clinical pharmacy services in Northern Sweden: a qualitative study. BMC Health Serv Res. 2018;18:35. doi:10.1186/s12913-018-2841-3
41. da Silva BA, Krishnamurthy M. The alarming reality of medication error: a patient case and review of Pennsylvania and National data. J Community Hosp Intern Med Perspect. 2016;6(4):31758. doi:10.3402/jchimp.v6.31758
42. Abousheishaa AA, Sulaiman AH, Huri HZ, Zaini S, Othman NA, Aladdin Zb, et al. Global Scope of Hospital Pharmacy Practice: A Scoping Review. Healthcare. 2020;8(2):143. doi:10.3390/healthcare8020143
43. Alfadl AA, Alrasheedy AA, Alhassun MS. Evaluation of medication counseling practice at community pharmacies in Qassim region, Saudi Arabia. Saudi Pharm J. 2018;26(2):258-62. doi:10.1016/j.jsps.2017.12.002
44. Jacobi J. Clinical Pharmacists: Practitioners Who Are Essential Members of Your Clinical Care Team. Rev Méd Clín Las Condes. 2016;27(5):571-7. doi:10.1016/j.rmclc.2016.09.002
45. Boostani K, Noshad H, Farnood F, Rezabee H, Teimouri S, Entezari-Maleki T, et al. Detection and Management of Common Medication Errors in Internal Medicine Wards: Impact on Medication Costs and Patient Care. Adv Pharm Bull. 2019;9(1):174-9. doi:10.15171/apb.2019.020
46. Alqenae FA, Steinke D, Keers RN. Prevalence and Nature of Medication Errors and Medication-Related Harm Following Discharge from Hospital to Community Settings: A Systematic Review. Drug Saf. 2020;43(6):517-37. doi:10.1007/s40264-020-00918-3
47. Keers RN, Williams SD, Cooke J, Ashcroft DM. Causes of Medication Administration Errors in Hospitals: a Systematic Review of Quantitative and Qualitative Evidence. Drug Saf. 2013;36(11):1045-67. doi:10.1007/s40264-013-0090-2
48. Cheragi MA, Manoocheri H, Mohammadnejad E, Ehsani SR. Types and causes of medication errors from nurse's viewpoint. Iran J Nurs Midwifery Res. 2013;18(3):228-31.
49. Zhou S, Kang H, Yao B, Gong Y. Analyzing Medication Error Reports in Clinical Settings: An Automated Pipeline Approach. AMIA Annu Symp Proc. 2018;2018:1611-20.
50. Tan X, Gu D, Lin X, Fang H, Asakawa T. Investigation of the characteristics of medication errors and adverse drug reactions using pharmacovigilance data in China. Saudi Pharm J. 2020;28(10):1190-6. doi:10.1016/j.jsps.2020.08.008
51. Jin J, Sklar GE, Oh VMS, Li SC. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269-86. doi:10.2147/tcrm.s1458
52. Mishra SI, Gioia D, Childress S, Barnet B, Webster RL. Adherence to Medication Regimens among Low-Income Patients with Multiple Comorbid Chronic Conditions. Health Soc Work. 2011;36(4):249-58. doi:10.1093/hsw/36.4.249
53. de Magalhães AMM, de Moura GMSS, Pasin SS, Funcke LB, Pardal BM, Kreling A. The medication process, workload and patient safety in inpatient units. Rev Esc Enferm USP. 2015;49:43-50. doi:10.1590/s0080-623420150000700007
54. McLellan AT. Substance Misuse and Substance use Disorders: Why do they Matter in Healthcare? Trans Am Clin Climatol Assoc. 2017;128:112-30.
55. Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11-22. doi:10.1177/2042098615615472
56. Hosain F, Lee J, Ata A, Bhullar RK, Chang AK. Physician Renewal of Chronically Prescribed Controlled Substances Based on Urine Drug Test Results. J Prim Care Community Health. 2019;10:2150132719883632. doi:10.1177/2150132719883632
57. Misasi P, Keebler JR. Medication safety in emergency medical services: approaching an evidence-based method of verification to reduce errors. Ther Adv Drug Saf. 2019;10:2042098618821916. doi:10.1177/2042098618821916
58. Velo GP, Minuz P. Medication errors: prescribing faults and prescription errors. Br J Clin Pharmacol. 2009;67(6):624-8. doi:10.1111/j.1365-2125.2009.03425.x
59. Bondesson A, Eriksson T, Kragh A, Holmdahl L, Midlöv P, Höglund P. In-hospital medication reviews reduce unidentified drug-related problems. Eur J Clin Pharmacol. 2013;69(3):647-55. doi:10.1007/s00228-012-1368-5
60. Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP. 1990;24(11):1093-7. doi:10.1177/106002809002401114
61. Garin N, Sole N, Lucas B, Matas L, Moras D, Rodrigo-Troyano A, et al. Drug related problems in clinical practice: a cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci Rep. 2021;11:883. doi:10.1038/s41598-020-80560-2
62. Westerlund T, Marklund B. Community pharmacy and primary health care in Sweden - at a crossroads. Pharm Pract. 2020;18(2):1927. doi:10.18549/PharmPract.2020.2.1927
63. Giardina C, Cutroneo PM, Mocciaro E, Russo GT, Mandraffino G, Basile G, et al. Adverse Drug Reactions in Hospitalized Patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study. Front Pharmacol. 2018;9:350. doi:10.3389/fphar.2018.00350
64. Molina-Mula J, Gallo-Estrada J. Impact of Nurse-Patient Relationship on Quality of Care and Patient Autonomy in Decision-Making. Int J Environ Res Public Health. 2020;17(3):835. doi:10.3390/ijerph17030835
65. Bramer WM, de Jonge GB, Rethlefsen ML, Mast F, Kleijnen J. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. 2018;106(4):531-41. doi:10.5195/jmla.2018.283
66. Green BN, Johnson CD. Interprofessional collaboration in research, education, and clinical practice: working together for a better future. J Chiropr Educ. 2015;29(1):1-10. doi:10.7899/JCE-14-36
67. Aubert CE, Streit S, Da Costa BR, Collet TH, Cornuz J, Gaspoz JM, et al. Polypharmacy and specific comorbidities in university primary care settings. Eur J Intern Med. 2016;35:35-42. doi:10.1016/j.ejim.2016.05.022
68. Payne RA, Abel GA, Avery AJ, Mercer SW, Roland MO. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol. 2014;77(6):1073-82. doi:10.1111/bcp.12292
69. Hailu BY, Berhe DF, Gudina EK, Gidey K, Getachew M. Drug related problems in admitted geriatric patients: the impact of clinical pharmacist interventions. BMC Geriatr. 2020;20:13. doi:10.1186/s12877-020-1413-7
70. Paisansirikul A, Ketprayoon A, Ittiwattanakul W, Petchlorlian A. Prevalence and Associated Factors of Drug-Related Problems Among Older People: A Cross-Sectional Study at King Chulalongkorn Memorial Hospital in Bangkok. Drugs Real World Outcomes. 2021;8(1):73-84. doi:10.1007/s40801-020-00219-2
71. Ramananth KV, Nedumbaili S. Assessment of Medication-Related Problems in Geriatric Patients of a Rural Tertiary Care Hospital. J Young Pharm. 2012;4(4):273-8. doi:10.4103/0975-1483.104372
72. Nobili A, Garattini S, Mannucci PM. Multiple diseases and polypharmacy in the elderly: challenges for the internist of the third millennium. J Comorb. 2011;1:28-44. doi:10.15256/joc.2011.1.4
73. Kurt M, Akdeniz M, Kavukcu E. Assessment of Comorbidity and Use of Prescription and Nonprescription Drugs in Patients Above 65 Years Attending Family Medicine Outpatient Clinics. Gerontol Geriatr Med. 2019;5:2333721419874274. doi:10.1177/2333721419874274
74. Peterson C, Gustafsson M. Characterisation of Drug-Related Problems and Associated Factors at a Clinical Pharmacist Service-Naïve Hospital in Northern Sweden. Drugs Real World Outcomes. 2017;4(2):97-107. doi:10.1007/s40801-017-0108-7
75. Gerber W, Steyn JD, Kotzé AF, Hamman JH. Beneficial Pharmacokinetic Drug Interactions: A Tool to Improve the Bioavailability of Poorly Permeable Drugs. Pharmaceutics. 2018;10(3):106. doi:10.3390/pharmaceutics10030106
76. Palleria C, Di Paolo A, Giofrè C, Caglioti C, Leuzzi G, Siniscalchi A, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. 2013;18(7):601-10.
77. Svensson EM, Acharya C, Clauson B, Dooley KE, Karlsson MO. Pharmacokinetic Interactions for Drugs with a Long Half-Life—Evidence for the Need of Model-Based Analysis. AAPS J. 2016;18(1):171-9. doi:10.1208/s12248-015-9829-2
78. Cascorbi I. Drug Interactions—Principles, Examples and Clinical Consequences. Dtsch Arztebl Int. 2012;109(33-34):546-56. doi:10.3238/arztebl.2012.0546
79. De Rose DU, Cairoli S, Dionisi M, Santisi A, Massenzi L, Goffredo BM, et al. Therapeutic Drug Monitoring Is a Feasible Tool to Personalize Drug Administration in Neonates Using New Techniques: An Overview on the Pharmacokinetics and Pharmacodynamics in Neonatal Age. Int J Mol Sci. 2020;21(16):5898. doi:10.3390/ijms21165898
80. McDonagh EM, Boukouvala S, Aklillu E, Hein DW, Altman RB, Klein TE. PharmGKB Summary: Very Important Pharmacogene information for N-acetyltransferase 2. Pharmacogenet Genomics. 2014;24(8):409-25. doi:10.1097/FPC.0000000000000062
81. Jacob S, Nair AB. An Updated Overview on Therapeutic Drug Monitoring of Recent Antiepileptic Drugs. Drugs R&D. 2016;16:303-16. doi:10.1007/s40268-016-0148-6
82. Tobias JD, Leder M. Procedural sedation: A review of sedative agents, monitoring, and management of complications. Saudi J Anaesth. 2011;5(4):395-410. doi:10.4103/1658-354X.87270
83. Shanika LGT, Wijekoon CN, Jayamanne S, Coombes J, Coombes I, Mamunuwa N, et al. Acceptance and attitudes of healthcare staff towards the introduction of clinical pharmacy service: a descriptive cross-sectional study from a tertiary care hospital in Sri Lanka. BMC Health Serv Res. 2017;17:46. doi:10.1186/s12913-017-2001-1
84. Ates HC, Roberts JA, Lipman J, Cass AEG, Urban GA, Dincer C. On-Site Therapeutic Drug Monitoring. Trends Biotechnol. 2020;38(11):1262-77. doi:10.1016/j.tibtech.2020.03.001
85. Feghali M, Venkataraman R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-9. doi:10.1053/j.semperi.2015.08.003
86. Johnson-Davis K, Doyle K. Therapeutic Drug Monitoring in Pregnant Patients. Ther Drug Monit. 2020;42(2):172-80. doi:10.1097/FTD.0000000000000709
87. Kristensen N, Nymann C, Konradsen H. Implementing research results in clinical practice- the experiences of healthcare professionals. BMC Health Serv Res. 2016;16:48. doi:10.1186/s12913-016-1292-y
88. Bank L, Jippes M, Scherpbier AJJA, den Rooyen C, Scheele F. How to Get Your Clinical Teaching Team Ready for Curriculum Change: A Practical Guide. Adv Med Educ Pract. 2019;10:979-86. doi:10.2147/AMEP.S211958
89. Childers CP, Maggard-Gibbons M. Understanding Costs of Care in the Operating Room. JAMA Surg. 2018;153(4):e176233. doi:10.1001/jamasurg.2017.6233
90. Harper L, Powell J, Pijl EM. An overview of forensic drug testing methods and their suitability for harm reduction point-of-care services. Harm Reduct J. 2017;14:52. doi:10.1186/s12954-017-0179-5
91. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. doi:10.1038/nrdp.2016.45
92. Lam WY, Fresco P. Medication Adherence Measures: An Overview. Biomed Res Int. 2015;2015:217047. doi:10.1155/2015/217047
93. Bronkhorst E, Gous AGS, Schellack N. Practice Guidelines for Clinical Pharmacists in Middle to Low Income Countries. Front Pharmacol. 2020;11:978. doi:10.3389/fphar.2020.00978
94. Bawankar RD, Mundhadad R, Wake PS, Kedia AS. Pharmacovigilance and Drug Safety in India - An Unmet Need or Challenge? J Pharmacovigil Pharmacother. 2017;17(5):1-3. doi:10.29011/JPPT-124.100024
95. Desai MK. Pharmacovigilance and assessment of drug safety reports during COVID 19. Perspect Clin Res. 2020;11(3):128-31. doi:10.4103/picr.PICR_171_20
96. Vallano A, Cereza G, Pedròs C, Agustí A, Danés I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60(6):653-8. doi:10.1111/j.1365-2125.2005.02504.x
97. Bhattacharya S. The Facts About Penicillin Allergy: A Review. J Adv Pharm Technol Res. 2010;1(1):11-7.
98. Sharif-Askari FS, Sharif-Askari NS, Javadi M, Gholami K. Adverse drug reactions reported to the drug and poison information center of Tehran, Iran. PLoS One. 2017;12(9):e0185450. doi:10.1371/journal.pone.0185450
99. Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379-407. doi:10.2165/00002018-200730050-00003
100. Ranganathan SS, Houghton JE, Davies DP, Routledge PA. The involvement of nurses in reporting suspected adverse drug reactions: experience with the meningococcal vaccination scheme. Br J Clin Pharmacol. 2003;56(6):658-63. doi:10.1046/j.1365-2125.2003.01903.x
101. Barolet D, Benohanian A. Current trends in needle-free jet injection: an update. Clin Cosmet Investig Dermatol. 2018;11:231-38. doi:10.2147/CCID.S162724
102. Ravi AD, Sadhna D, Nagpaal D, Chawla L. Needle free injection technology: A complete insight. Int J Pharm Investig. 2015;5(4):192-9. doi:10.4103/2230-973X.167662
Authors
Copyright (c) 2021 Gaurav Joshi, Atul Kabra, Nishant Goutam, Alka Sharma

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.