Iron-Overload Conditions: Manifestations to the Kidney Organs – A Review
Abstract
Excess iron is a risk factor for organ dysfunction and damage resulting in various organ diseases such as liver, heart, and kidney, diabetes mellitus, and neurodegenerative diseases. Iron overload in some individuals is caused by various factors, including genetic predisposition such as genetic hemochromatosis, repeated transfusion of red blood cells, and parenteral iron administration in conditions of transfusion-dependent anemia. A disturbance in the globin gene in diseases such as β-thalassemia major causes an imbalance of the globin chain, resulting in chronic anemia in the sufferer. It has been reported that the human body does not have a mechanism for eliminating excess iron levels. Routine transfusion has become a solution to overcome chronic anemia so that patients can maintain hemoglobin levels, and the result of this transfusion repetition is the accumulation of iron in various organs, such as the heart, liver, endocrine glands, pancreas, lungs, and kidneys. Excess iron can be toxic to the body due to the formation of harmful free radicals that can damage cells and tissues. An increase in excessive ROS can result in the saturation of the antioxidant system. The presence of free radicals can lead to damage and the occurrence of filtration dysfunction in the glomerulus.
Full text article
References
2. Udani K, Chris-Olaiya A, Ohadugha C, Malik A, Sansbury J, Paari D. Cardiovascular manifestations in hospitalized patients with hemochromatosis in the United States. Int J Cardiol. 2021;342:117-24. doi:10.1016/j.ijcard.2021.07.060
3. Laksmitawati DR, Handayani S, Udyaningsih-Freisleben SK, Kurniati V, Adhiyanto C, Hidayat J, et al. Iron status and oxidative stress in β-thalassemia patients in Jakarta. Biofactors. 2003;19(1–2):53–62. doi:10.1002/biof.5520190107
4. de Lima TG, Benevides FLN, Filho FLE, Farias IS, Dourado DXC, Fontenele EGP, et al. Treatment of iron overload syndrome: A general review. Rev Assoc Med Bras. 2019;65(9):1216–22. doi:10.1590/1806-9282.65.9.1216
5. Estuningtyas A, Wahyuni T, Wahidiyat PA, Poerwaningsih EH, Freisleben HJ. Mangiferin and mangiferin-containing leaf extract from Mangifera foetida L for therapeutic attenuation of experimentally induced iron overload in a rat model. J Herbmed Pharmacol. 2019;8(1):21–7. doi:10.15171/jhp.2019.04
6. Taher AT, Cappellini MD. Luspatercept for β-thalassemia: beyond red blood cell transfusions. Expert Opin Biol Ther. 2021;21(11):1363-71. doi:10.1080/14712598.2021.1968825
7. Sumneang N, Siri-Angkul N, Kumfu S, Chattipakorn SC, Chattipakorn N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch Biochem Biophys. 2020;680:108241. doi:10.1016/j.abb.2019.108241
8. Estuningtyas A, Setiabudy R, Wahidiyat PA, Freisleben HJ. The Role of Mangiferin in the Prevention of Experimentally Induced Iron Overload in an Animal Model. Drug Res. 2019;69(4):234–40. doi:10.1055/a-0667-8530
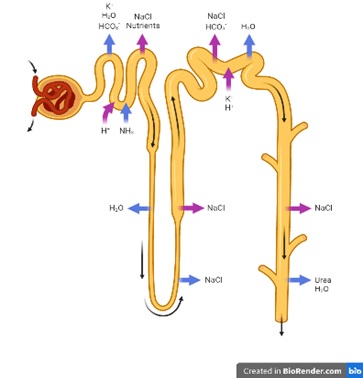
9. Bhandari S, Galanello R. Renal aspects of thalassaemia a changing paradigm. Eur J Haematol. 2012;89(3):187–97. doi:10.1111/j.1600-0609.2012.01819.x
10. Ashoor IF, Somers MJG. Physiology of the Developing Kidney: Fluid and Electrolyte Homeostasis and Therapy of Basic Disorders (Na/H2O/K/Acid Base). In: Avner E, Harmon W, Niaudet P, Yoshikawa N, Emma F, Goldstein S, editors. Pediatric Nephrology.Berlin, Heidelberg: Springer; 2015. doi:10.1007/978-3-642-27843-3_12-1
11. Sherwood L. Human physiology : from cells to systems. 7th edition. Belmont, CA: Brooks/Cole; 2013. p. 148–162.
12. Radi ZA. Kidney Pathophysiology, Toxicology, and Drug-Induced Injury in Drug Development. Int J Toxicol. 2019;38(3):215-27. doi:10.1177/1091581819831701
13. Brissot P, Troadec MB, Loréal O, Brissot E. Pathophysiology and classification of iron overload diseases; update 2018. Transfus Clin Biol. 2019;26(1):80–8. doi:10.1016/j.tracli.2018.08.006
14. Franke GN, Kubasch AS, Cross M, Vucinic V, Platzbecker U. Iron overload and its impact on outcome of patients with hematological diseases. Mol Aspects Med. 2020;75:100868. doi:10.1016/j.mam.2020.100868
15. Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21(Suppl 1):S6–20. doi:10.1111/hdi.12542
16. Li Y, Huang X, Wang J, Huang R, Wan D, Yang G. Regulation of Iron Homeostasis and Related Diseases. Mediators Inflamm. 2020;2020:6062094. doi:10.1155/2020/6062094
17. Sangkhae V, Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv Nutr. 2017;8(1):126–36. doi:10.3945/an.116.013961
18. Zeidan RS, Han SM, Leeuwenburgh C, Xiao R. Iron homeostasis and organismal aging. Ageing Res Rev. 2021;72:101510. doi:10.1016/j.arr.2021.101510
19. Mehta KJ, Je Farnaud S, Sharp PA. Iron and liver fibrosis: Mechanistic and clinical aspects. World J Gastroenterol. 2019;25(5):521–38. doi:10.3748/wjg.v25.i5.521
20. Shah FT, Sayani F, Trompeter S, Drasar E, Piga A. Challenges of blood transfusions in β-thalassemia. Blood Rev. 2019;37:100588. doi:10.1016/j.blre.2019.100588
21. Cakan P, Yildiz S, Akyay A, Öncül Y. Erythrocyte transfusion restored heart rate variability in children with thalassemia major. Transfus Apher Sci. 2021;60(4):103156. doi:10.1016/j.transci.2021.103156
22. Ho WL, Chung KP, Yang SS, Lu MY, Jou ST, Chang HH, et al. A pharmaco-economic evaluation of deferasirox for treating patients with iron overload caused by transfusion-dependent thalassemia in Taiwan. J Formos Med Assoc. 2013;112(4):221–9. doi:10.1016/j.jfma.2011.08.020
23. Ponticelli C, Musallam KM, Cianciulli P, Cappellini MD. Renal complications in transfusion-dependent beta thalassaemia. Blood Rev. 2010;24(6):239–44. doi:10.1016/j.blre.2010.08.004
24. Tanous O, Azulay Y, Halevy R, Dujovny T, Swartz N, Colodner R, et al. Renal function in β-thalassemia major patients treated with two different iron-chelation regimes. BMC Nephrol. 2021;22(1):418. doi:10.1186/s12882-021-02630-5
25. Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34(6):975–91. doi:10.1007/s00467-018-4005-4
26. Jha JC, Banal C, Chow BSM, Cooper ME, Jandeleit-Dahm K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxidants Redox Signal. 2016;25(12):657–84. doi:10.1089/ars.2016.6664
27. Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clin Biochem. 2011;44(14–15):1189–98. doi:10.1016/j.clinbiochem.2011.06.988
28. Griffin BR, Faubel S, Edelstein CL. Biomarkers of Drug-Induced Kidney Toxicity. Ther Drug Monit. 2019;41(2):213-26. doi:10.1097/ftd.0000000000000589
29. Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, et al. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int Rep. 2021;6(7):1775-87. doi:10.1016/j.ekir.2021.04.023
30. Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling ariadne’s thread. Int J Mol Sci. 2019;20(15):3711. doi:10.3390/ijms20153711
31. Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23(3):95–104. doi:10.1016/j.blre.2008.08.001
32. Kuragano T, Joki N, Hase H, Kitamura K, Murata T, Fujimoto S, et al. Low transferrin saturation (TSAT) and high ferritin levels are significant predictors for cerebrovascular and cardiovascular disease and death in maintenance hemodialysis patients. PLoS One. 2020;15(9):e0236277. doi:10.1371/journal.pone.0236277
33. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1(Suppl 1):S4–8. doi:10.2215/cjn.01490506
34. Gowda S, Desai PB, Kulkarni SS, Hull V V, Math AAK, Vernekar SN. Markers of renal function tests. N Am J Med Sci. 2010;2(4):170–3.
35. Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Thienpont LM, et al. Creatinine measurement: State of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129(3):297–304. doi:10.5858/2005-129-297-cmsota
36. Ige AO, Ongele FA, Adele BO, Emediong IE, Odetola AO, Adewoye EO. Pathophysiology of iron overload-induced renal injury and dysfunction: Roles of renal oxidative stress and systemic inflammatory mediators. Pathophysiology. 2019;26(2):175–80. doi:10.1016/j.pathophys.2019.03.002
37. Beheshti F, Norouzi F, Abareshi A, Khazaei M, Alikhani V. Nigella sativa Prevented Liver and Renal Tissue Damage in Lipopolysaccharide-Treated Rats. Saudi J Kidney Dis Transpl. 2018;29(3):554–66. doi:10.4103/1319-2442.235184
38. Ali BH, Al-Salam S, Al Suleimani Y, Al Kalbani J, Al Bahlani S, Ashique M, et al. Curcumin Ameliorates Kidney Function and Oxidative Stress in Experimental Chronic Kidney Disease. Basic Clin Pharmacol Toxicol. 2018;122(1):65–73. doi:10.1111/bcpt.12817
39. Zhang WR, Parikh CR. Biomarkers of Acute and Chronic Kidney Disease. Annu Rev Physiol. 2019;81:309–33. doi:10.1146/annurev-physiol-020518-114605
40. Verma PK, Raina R, Sultana M, Singh M, Kumar P. Total antioxidant and oxidant status of plasma and renal tissue of cisplatin-induced nephrotoxic rats: Protection by floral extracts of Calendula officinalis Linn. Ren Fail. 2016;38(1):142–50. doi:10.3109/0886022x.2015.1103585
41. Aguilar TAF, Navarro BCH, Pérez JAM. Endogenous Antioxidants: A Review of their Role in Oxidative Stress. In: Morales-Gonzalez JA, Morales-Gonzalez A, Madrigal-Santillan EO, editors. A Master Regulator of Oxidative Stress - The Transcription Factor Nrf2. London, UK: IntechOpen; 2016. doi:10.5772/65715
42. Todorova I, Simeonova G, Kyuchukova D, Dinev D, Gadjeva V. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Path. 2005;13(4):190–4. doi:10.1007/s00580-005-0547-5
43. Pérez-Morales RE, Del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, Navarro-González JF. Inflammation in diabetic kidney disease. Nephron. 2019;143(1):12–6. doi:10.1159/000493278
44. Guarda NS, Bollick YS, De Carvalho JAM, Premaor MO, Comim FV, Moresco RN. High serum uric acid is associated with tubular damage and kidney inflammation in patients with type 2 diabetes. Dis Markers. 2019;2019:6025804. doi:10.1155/2019/6025804
Authors
Copyright (c) 2023 Nadia Larasinta Heriatmo, Ari Estuningtyas, Vivian Soetikno

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.