Trend in the Utilization of Antipsychotics in the National Health Coverage Era in Indonesia: A Cross-Sectional Study
Abstract
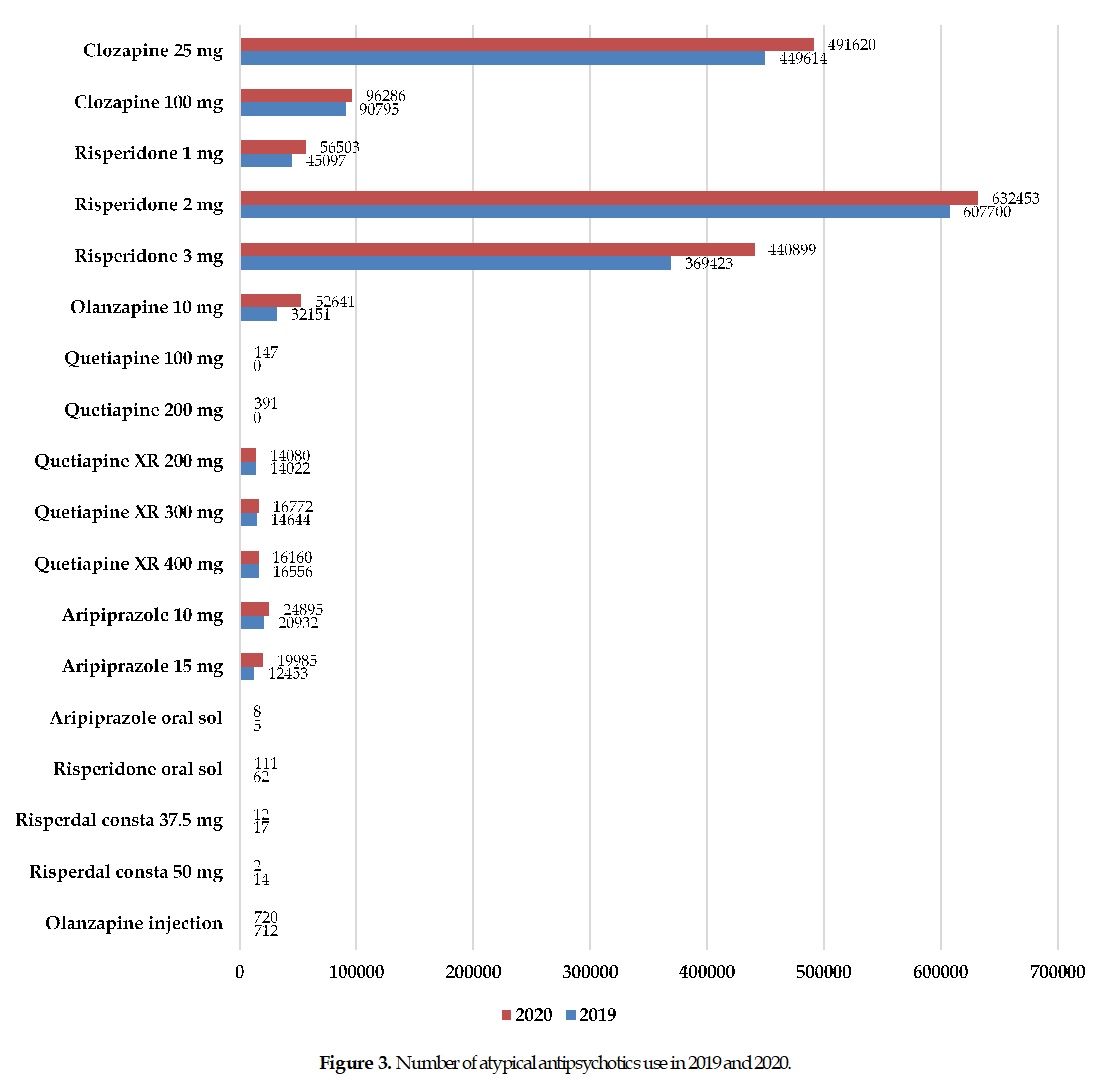
The utilization pattern of antipsychotics has undergone significant changes since the introduction of atypical antipsychotics. Currently, medication for patients with schizophrenia predominantly uses atypical antipsychotics rather than typical antipsychotics. This study aimed to present the updated utilization pattern of antipsychotics among Indonesians. A cross-sectional study was conducted in 2019-2020 at the National Mental Hospital in Indonesia. Data were collected from medication-used reports from either inpatients or outpatients. A descriptive analysis was conducted to present the pattern and the annual total cost for each antipsychotic used. The pattern of typical antipsychotics used from 2019 to 2020 was likely to decline. The total cost estimated for typical antipsychotics in 2019 was IDR 475 million, and IDR 420 million in 2020. Trifluoperazine 5 mg was the most commonly typical antipsychotic used, followed by chlorpromazine 100 mg and haloperidol 5 mg. Eventually, the pattern of atypical antipsychotics used was likely to increase. The total cost was estimated at IDR 3.2 billion in 2019 and IDR 3.8 billion in 2020. Risperidone 2 mg was the most commonly atypical antipsychotic used, followed by clozapine 25 mg and risperidone 3 mg. This study proves the trend toward increased atypical antipsychotics used. Accordingly, the cost of schizophrenia treatment was elevated.
Full text article
References
2. Zhu B, Svanum HA, Faries DE, Correll CU, Kane JM. Cost of antipsychotic polypharmacy in the treatment of schizophrenia. BMC Psychiatry. 2008;8:9. DOI: 10.1186/1471-244X-8-19; PMCID: PMC2364616; PMID: 18394168
3. Sweileh WM, Odeh JB, Shraim NY, Zyoud SH, Sawalha AF, Al-Jabi SW. Evaluation of Defined Daily Dose, percentage of British National Formulary maximum and chlorpromazine equivalents in antipsychotic drug utilization. Saudi Pharm J. 2014;22(2):127–32. DOI: 10.1016/j.jsps.2013.03.003; PMCID: PMC3950502; PMID: 24648824
4. Patted UH, Hema NG, Nagaraj A. Antipsychotics in schizophrenia: a retrospective study of drug utilization pattern in outpatient department of psychiatry at a tertiary care hospital. Int J Basic Clin Pharmacol. 2017;7(1):167-72. DOI: 10.18203/2319-2003.ijbcp20175694
5. Fabrazzo M, Cipolla S, Camerlengo A, Perris F, Catapano F. Second-Generation Antipsychotics' Effectiveness and Tolerability: A Review of Real-World Studies in Patients with Schizophrenia and Related Disorders. J Clin Med. 2022;11(15):4530. DOI: 10.3390/jcm11154530; PMCID: PMC9369504; PMID: 35956145
6. Berger A, Edelsberg J, Sanders KN, Alvir JM, Mychaskiw MA, Oster G. Medication adherence and utilization in patients with schizophrenia or bipolar disorder receiving aripiprazole, quetiapine, or ziprasidone at hospital discharge: a retrospective cohort study. BMC Psychiatry. 2012;12:99. DOI: 10.1186/1471-244X-12-99; PMCID: PMC3480886; PMID: 22856540
7. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs, 2011;25(5):383–99. DOI: 10.2165/11587810-000000000-00000; PMID: 21476610
8. Nappoe SA, Djasri H, Kurniawan MF. Chronic disease management programme (PROLANIS) in Indonesia: case study. Geneva: World Health Organization, Organisation for Economic Co-operation and Development; 2023.
9. Kadakia A, Catillon M, Fan Q, Williams GR, Marden JR, Anderson A, et al. The Economic Burden of Schizophrenia in the United States. J Clin Psychiatry. 2022;83(6):22m14458. DOI: 10.4088/jcp.22m14458; PMID: 36244006
10. Puspitasari IM, Sinuraya RK, Rahayu C, Witriani W, Zannah U, Hafifah A, et al. Medication Profile and Treatment Cost Estimation Among Outpatients with Schizophrenia, Bipolar Disorder, Depression, and Anxiety Disorders in Indonesia. Neuropsychiatr Dis Treat. 2020;16:815-28. DOI: 10.2147/NDT.S240058
11. Piparva KG, Parmar DM, Singh AP, Gajera MV, Trivedi HR. Drug utilization study of psychotropic drugs in outdoor patients in a teaching hospital. Indian J Psychol Med. 2011;33(1):54–8. DOI: 10.4103/0253-7176.85396; PMCID: PMC3195156; PMID: 22021954
12. Deshmukh S, Ismail T. Evaluation of Psychotropic Drugs Use Pattern Among Out Patients Attending Psychiatry Department at Government Medical College And Hospital, Nagpur: A Cross Sectional Study. Int J Pharm Bio Sci. 2012;3(3): 428-36.
13. Wold Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-4. DOI: 10.1001/jama.2013.281053; PMID: 24141714
14. Thakkar KB, Jain MM, Billa G, Joshi A, Khobragade AA. A drug utilization study of psychotropic drugs prescribed in the psychiatry outpatient department of a tertiary care hospital. J Clin Diagn Res. 2013;7(12):2759-64. DOI: 10.7860/jcdr/2013/6760.3885; PMCID: PMC3919294; PMID: 24551631
15. Mohamed MMA, Yousef BA. Prescription patterns of antipsychotics in the management of first episode psychosis at three psychiatric hospitals in Khartoum, 2018: A descriptive cross-sectional study. J Family Med Prim Care. 2020;9(1):402–6. DOI: 10.4103/jfmpc.jfmpc_892_19; PMCID: PMC7014859; PMID: 32110626
16. Shaifali I, Karmakar R, Chandra S, Kumar S. Drug utilization audit of antipsychotics using WHO methodology: recommendations for rational prescribing. Int J Basic Clin Pharmacol. 2018;7(10):2021-7. DOI: 10.18203/2319-2003.ijbcp20183941
17. Cheung S, Hamuro Y, Mahlich J, Nakahara T, Sruamsiri R, Tsukazawa S. Drug Utilization of Japanese Patients Diagnosed with Schizophrenia: An Administrative Database Analysis. Clin Drug Investig. 2017;37(6):559–69. DOI: 10.1007/s40261-017-0517-0; PMCID: PMC5422449; PMID: 28361438
18. Lee L, Procyshyn RM, White RF, Woodward TS, Honer WG, & Barr AM. Antipsychotic prescribing patterns on admission to and at discharge from a tertiary care program for treatment-resistant psychosis. PLoS One. 2018;13(8):e0199758. DOI: 10.1371/journal.pone.0199758; PMCID: PMC6086406; PMID: 30096136
19. Roberto PN, Brandt N, Onukwugha E, Perfetto E, Powers C, Stuart B. The Impact of Coverage Restrictions on Antipsychotic Utilization Among Low-Income Medicare Part D Enrollees. Adm Policy Ment Health. 2017;44(6):943–54. DOI: 10.1007/s10488-017-0813-2; PMID: 28660370
20. Dipiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: a pathophysiologic approach. 10th edition. New York: Mc Graw-Hill Medical Publishing Division; 2017.
21. Park SC, Lee MS, Kang SG, Lee SH. Patterns of antipsychotic prescription to patients with schizophrenia in Korea: results from the health insurance review & assessment service-national patient sample. J Korean Med Sci. 2014;29(5):719–28. DOI: 10.3346/jkms.2014.29.5.719; PMCID: PMC4024938; PMID: 24851031
22. Gaviria AM, Franco J G, Aguado V, Rico G, Labad J, de Pablo J, et al. A Non-Interventional Naturalistic Study of the Prescription Patterns of Antipsychotics in Patients with Schizophrenia from the Spanish Province of Tarragona. PLoS One. 2015;10(10):e0139403. DOI: 10.1371/journal.pone.0139403; PMCID: PMC4591292; PMID: 26427051
23. Mahmood S, Hussain S, Rehman TU, Barbui C, Kurdi AB, Godman B. Trends in the prescribing of antipsychotic medicines in Pakistan: implications for the future. Curr Med Res Opin. 2018;35(1):51–61. DOI: 10.1080/03007995.2018.1513834; PMID: 30122062
24. Julaeha J, Athiyah U, & Hermansyah A. The prescription patterns of second-generation antipsychotics in schizophrenia outpatient setting. J Basic Clin Physiol Pharmacol. 2019;30:(6):20190289. DOI: 10.1515/jbcpp-2019-0289; PMID: 31837257
25. Yazici E, Cilli AS, Yazici AB, Baysan H, Ince M, Bosgelmez S, et al. Antipsychotic Use Pattern in Schizophrenia Outpatients: Correlates of Polypharmacy. Clin Pract Epidemiol Ment Health. 2017;13:92–103. DOI: 10.2174/1745017901713010092; PMCID: PMC5633702; PMID: 29081826
26. Kim HY, Lee HW, Jung SH, Kang MH, Bae JN, Lee JS, et al. Prescription patterns for patients with schizophrenia in Korea: a focus on antipsychotic polypharmacy. Clin Psychopharmacol Neurosci. 2014;12(2):128–36. DOI: 10.9758/cpn.2014.12.2.128; PMCID: PMC4153859; PMID: 25191503
27. Julaeha J, Athiyah U, Yuliana V, Ayuningtyas JP, Hermansyah A. Revisiting the intractable barriers affecting medication adherence among outpatients with schizophrenia. Curr Trends Biotechnol Pharm. 2020;14(5):200-5. DOI: 10.5530/ctbp.2020.4s.24
28. Díaz-Castro L, Cabello-Ranger L, Arredondo A, de León EM, Pineda-Antúnez C. Cost-effectiveness of therapeutic interventions in schizophrenia. Eur J Psychiatry. 2017;31(1):11-6. DOI: 10.1016/j.ejpsy.2016.12.006
29. Fan SJ, Lu N, Chang HC, Tang CH, Huang KC. Health service utilization and medical costs among patients with schizophrenia receiving long-acting injectable risperidone versus oral risperidone: A nationwide retrospective matched cohort study in Taiwan. Int Clin Psychopharmacol. 2018;.33(4):204-12. DOI: 10.1097/YIC.0000000000000213; PMID: 29489495
30. Yan T, Greene M, Chang E, Hartry A, Touya M, Broder MS. All-cause hospitalization and associated costs in patients with schizophrenia or bipolar disorder initiating long-acting injectable antipsychotics. Cur Med Res Opin. 2018;34(1):41–7. DOI: 10.1080/03007995.2017.1395733; PMID: 29057674
31. Munday J, Greene M, Chang E, Hartry A, Yan T, Broder MS. Early initiation of long-acting injectable antipsychotic treatment is associated with lower hospitalization rates and healthcare costs in patients with schizophrenia: real-world evidence from US claims data. Cur Med Res Opin. 2019;35(7):1231–9. DOI: 10.1080/03007995.2019.1571295; PMID: 30649965
32. Julaeha J, Athiyah U, Ayuningtyas JP, Yuliana V, Hermansyah A. Metabolic Syndrome Risk Associated with Atypical Antipsychotic Medication: A Case Report. J Drug Deliv Ther. 2021;11(1):77-9. DOI: 10.22270/jddt.v11i1.4680
Authors
Copyright (c) 2024 Julaeha Julaeha, Verra Yuliana, Josephine Paramita Ayuningtyas

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.