Potential Antidiabetic Compounds from Anogeissus leiocarpus: Molecular Docking, Molecular Dynamic Simulation, and ADMET Studies
Abstract
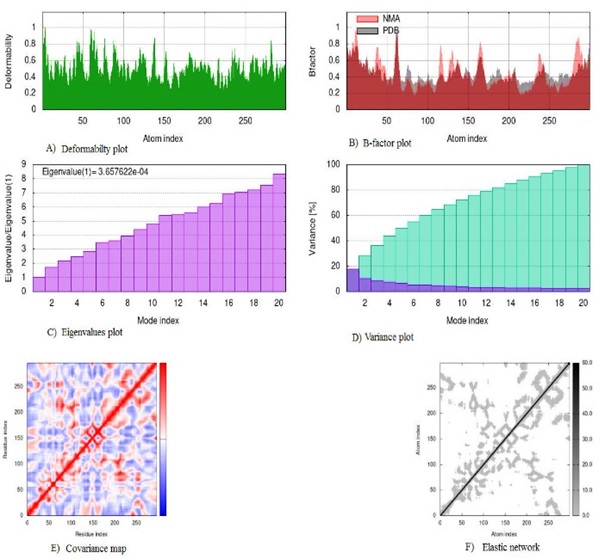
This study aimed to evaluate the antidiabetic potential of compounds from Anogeissus leiocarpus in silico and the potential of the compounds as antidiabetic drug candidates. Molecular docking (MD), molecular dynamics simulation (MDS), and ADMET were carried out in silico to evaluate the compounds' antidiabetic potential and drug candidacy. The MDS revealed the least BA (-8.7 kcal/mol) was exhibited by compound X (palmitic acid) with Glucagon-like Peptide-1 Receptor (GLP1), while the highest BA (-5.8 kcal/mol) was demonstrated by I (1,2,4-benzetriol) with dipeptidyl peptidase IV (DPP-4) among the best interactions. The MDS result showed good docked complexes' flexibility, deformability, and stability with low eigenvalues ranging from 8.52 × 10-5 to 1.30 × 10-4. All the compounds had a bioavailability score of 0.55 except VI (0.85), while the synthetic ability showed a good score of ≤3.01. Eight compounds were predicted to be soluble, with two poorly soluble. Additionally, all the compounds had high gastrointestinal absorption, with the majority being blood-brain barrier permeant, while skin permeation value was between -2.55 and -7.48 cm/s. Furthermore, none of the compounds were either permeability glycoprotein (P-gp) substrate or CYP2C19 and CYP2C9 inhibitors, though some were CYP1A2, CYP2D6, and CYP3A4 inhibitors. Moreover, the toxicity study showed moderate to non-toxicity results with toxicity classes between 3 and 5. Conclusively, the compounds from A. leiocarpus showed good binding interactions, which are the protein targets of antidiabetic therapy and potentially good candidates for antidiabetic drug development.
Full text article
References
2. Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020;10(4):174-88. doi:10.4103/ajm.ajm_53_20
3. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
4. Maiti S, Akhtar S, Upadhyay AK, Mohanty SK. Socioeconomic inequality in awareness, treatment and control of diabetes among adults in India: Evidence from National Family Health Survey of India (NFHS), 2019-2021. Sci Rep. 2023;13(1):2971. doi:10.1038/s41598-023-29978-y
5. Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(8):543-51. doi:10.7326/0003-4819-159-8-201310150-00007
6. American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S98-110. doi:10.2337/dc20-s009
7. American Diabetes Association. 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S34-9. doi:10.2337/dc21-s003
8. Dahiru MM, Samuel NM. A review of the Mechanisms of Action and Side Effects of Anti-diabetic Agents. Trends Pharm Sci. 2022;8(3):195-210. doi:10.30476/tips.2022.95931.1153
9. Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11(11):1185-200. doi:10.7150/ijms.10001
10. Mohammed A, Tajuddeen N. Antidiabetic compounds from medicinal plants traditionally used for the treatment of diabetes in Africa: A review update (2015–2020). South Afr J Bot. 2022;146:585-602. doi:10.1016/j.sajb.2021.11.018
11. Teoh SL, Das S. Phytochemicals and their effective role in the treatment of diabetes mellitus: a short review. Phytochem Rev. 2018;17(5):1111-28. doi:10.1007/s11101-018-9575-z
12. Mohammed S, Yaqub A, Sanda K, Nicholas A, Arastus W, Muhammad M, et al. Review on diabetes, synthetic drugs and glycemic effects of medicinal plants. J Med Plants Res. 2013;7(36):2628-37. doi:10.5897/JMPR2013.5169
13. Adhikari B. Roles of alkaloids from medicinal plants in the management of diabetes mellitus. J Chem. 2021;2021:2691525. doi:10.1155/2021/2691525
14. Negbenebor HE, Shehu K, Mukhtar M, Oiza A, Nura S, Fagwalaw L. Ethnobotanical survey of medicinal plants used by Hausa people in the management of diabetes mellitus in Kano metropolis, northern Nigeria. Eur J Med Plants. 2017;18(2):1-10. doi:10.9734/EJMP/2017/28562
15. Manukumar HM, Kumar JS, Chandrasekhar B, Raghava S, Umesha S. Evidences for diabetes and insulin mimetic activity of medicinal plants: present status and future prospects. Crit Rev Food Sci Nutr. 2017;57(12):2712-29. doi:10.1080/10408398.2016.1143446
16. Arbab A. Review on Anogeissus Leiocarpus a Potent African Traditional Drug. Int J Res Pharm Chem. 2014;4(3):496-500.
17. Salih EYA, Julkunen-Tiitto R, Luukkanen O, Sipi M, Fahmi MKM, Fyhrquist PJ. Potential anti-tuberculosis activity of the extracts and their active components of Anogeissus leiocarpa (Dc.) guill. and perr. with special emphasis on polyphenols. Antibiotics. 2020;9(7):364. doi:10.3390/antibiotics9070364
18. Mukhtar Y, Abdu K, Maigari AK. Efficacy of Anogeissus leiocarpus (DC.) as potential therapeutic agent against Trypanosomiasis diseases: A review. Int J Health Pharm Res. 2017;3(3):1-9.
19. Okpekon T, Yolou S, Gleye C, Roblot F, Loiseau P, Bories C, et al. Antiparasitic activities of medicinal plants used in Ivory Coast. J Ethnopharmacol. 2004;90(1):91-7. doi:10.1016/j.jep.2003.09.029
20. Barku VYA, Opoku-Boahen Y, Owusu-Ansah E, Dayie NTKD, Mensah FE. In-vitro assessment of antioxidant and antimicrobial activities of methanol extracts of six wound healing medicinal plants. J. Nat Sci Res. 2013;3(1):74-80.
21. Tauheed AM, Mamman M, Ahmed A, Suleiman MM, Balogun EO. In vitro and in vivo antitrypanosomal efficacy of combination therapy of Anogeissus leiocarpus, Khaya senegalensis and potash. J Ethnopharmacol. 2020;258:112805. doi:10.1016/j.jep.2020.112805
22. Tagne MAF, Rékabi Y, Noubissi PA, Fankem GO, Akaou H, Wambe H, et al. Evaluation of antidiarrheal activity of aqueous leaf extract of Anogeissus leiocarpus on castor oil-induced diarrhea in rats. Am J Biomed Sci Res. 2019;3(1):27-34. doi:10.34297/AJBSR.2019.03.000629
23. Dahiru MM, Abaka AM, Artimas SP. Phytochemical Analysis and Antibacterial Activity of Methanol and Ethyl Acetate Extracts of Detarium microcarpum Guill. & Perr. Bio Med Nat Prod Chem. 2023;12(1):281-8. doi:10.14421/biomedich.2023.121.281-288
24. Hassan LEA, Al-Suade FS, Fadul SM, Majid AMSA. Evaluation of antioxidant, antiangiogenic and antitumor properties of Anogeissus leiocarpus against colon cancer. J Angiotherapy. 2018;1(2):56-66. doi:10.25163/angiotherapy.1200021526100818
25. Motto AE, Lawson-Evi P, Bakoma B, Eklu-Gadegbeku K, Aklikokou K. Antihyperlipidemic and antioxidant properties of hydro-alcoholic extracts from Anogeissus leiocarpus (Combretaceae). Heliyon. 2021;7(4):e06648. doi:10.1016/j.heliyon.2021.e06648
26. Motto AE, Lawson-Evi P, Eklu-Gadegbeku K. Antidiabetic and antioxidant potential of total extract and supernatant fraction of the roots of Anogeissus leiocarpus in HFD-fed and Streptozocin-induced diabetic rats. Biomed Pharmacother. 2022;154:113578. doi:10.1016/j.biopha.2022.113578
27. Num-Adom SM, Adamu S, Aluwong T, Ogbuagu NE, Umar IA, Esievo KAN. Ethanolic extract of Anogeissus leiocarpus ameliorates hyperglycaemia, hepato-renal damage, deranged electrolytes and acid-base balance in alloxan-induced diabetes in dogs. Sci Afr. 2022;16:e01183. doi:10.1016/j.sciaf.2022.e01183
28. Dahiru MM, Badgal EB, Neksumi M. Phytochemical Profiling And Heavy Metals Composition Of Aqueous and Ethanol Extracts of Anogeissus Leiocarpus. J Fac Pharm Ankara Uni. 2023;47(2):311-23. doi:10.33483/jfpau.1205941
29. O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox. J Cheminform. 2011;3:33. doi:10.1186/1758-2946-3-33
30. Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17(1):57-61.
31. Pratama MRF, Sutomo S. Chemical Structure Optimization of Lupeol As ER-α and HER2 Inhibitor. Asian J Pharm Clin Res. 2018;11(6):298-303. doi:10.22159/ajpcr.2018.v11i6.24226
32. Adasme MF, Linnemann KL, Bolz SN, Kaiser F, Salentin S, Haupt VJ, et al. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021;49(W1):W530-4. doi:10.1093/nar/gkab294
33. Ortiz CLD, Completo GC, Nacario RC, Nellas RB. Potential Inhibitors of Galactofuranosyltransferase 2 (GlfT2): Molecular Docking, 3D-QSAR, and In Silico ADMETox Studies. Sci Rep. 2019;9(1):17096. doi:10.1038/s41598-019-52764-8
34. Kurcinski M, Oleniecki T, Ciemny MP, Kuriata A, Kolinski A, Kmiecik S. CABS-flex standalone: a simulation environment for fast modeling of protein flexibility. Bioinformatics. 2019;35(4):694-5. doi:10.1093/bioinformatics/bty685
35. López-Blanco JR, Aliaga JI, Quintana-Ortí ES, Chacón P. iMODS: internal coordinates normal mode analysis server. Nucleic Acids Res. 2014;42(W1):W271-6. doi:10.1093/nar/gku339
36. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):42717. doi:10.1038/srep42717
37. Banerjee P, Eckert AO, Schrey AK, Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018;46(W1):W257-63. doi:10.1093/nar/gky318
38. Tamrakar AK, Maurya CK, Rai AK. PTP1B inhibitors for type 2 diabetes treatment: a patent review (2011–2014). Expert Opin Ther Pat. 2014;24(10):1101-15. doi:10.1517/13543776.2014.947268
39. Hussain H, Green IR, Abbas G, Adekenov SM, Hussain W, Ali I. Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: patent review (2015-2018). Expert Opin Ther Pat. 2019;29(9):689-702. doi:10.1080/13543776.2019.1655542
40. Sobhia ME, Paul S, Shinde R, Potluri M, Gundam V, Kaur A, et al. Protein tyrosine phosphatase inhibitors: a patent review (2002–2011). Expert Opin Ther Pat. 2012;22(2):125-53. doi:10.1517/13543776.2012.661414
41. Wan ZK, Lee J, Hotchandani R, Moretto A, Binnun E, Wilson DP, et al. Structure-Based Optimization of Protein Tyrosine Phosphatase-1 B Inhibitors: Capturing Interactions with Arginine 24. ChemMedChem. 2008;3(10):1525-9. doi:10.1002/cmdc.200800188
42. Ghosh P, Bhakta S, Bhattacharya M, Sharma AR, Sharma G, Lee SS, et al. A novel multi-epitopic peptide vaccine candidate against Helicobacter pylori: in-silico identification, design, cloning and validation through molecular dynamics. Int J Pept Res Ther. 2021;27(2):1149-66. doi:10.1007/s10989-020-10157-w
43. Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94(8):2692-701. doi:10.1210/jc.2009-0370
44. Julian LD, Wang Z, Bostick T, Caille S, Choi R, DeGraffenreid M, et al. Discovery of Novel, Potent Benzamide Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD1) Exhibiting Oral Activity in an Enzyme Inhibition ex Vivo Model. J Med Chem. 2008;51(13):3953-60. doi:10.1021/jm800310g
45. Ferenczy GG, Kellermayer M. Contribution of hydrophobic interactions to protein mechanical stability. Comput StructBiotechnol J. 2022;20:1946-56. doi:10.1016/j.csbj.2022.04.025
46. Kume S, Uzu T, Isshiki K, Koya D. Peroxisome Proliferator-Activated Receptors in Diabetic Nephropathy. PPAR Res. 2008;2008:879523. doi:10.1155/2008/879523
47. Chiarelli F, Marzio DD. Peroxisome proliferator-activated receptor-γ agonists and diabetes: current evidence and future perspectives. Vasc Health Risk Manag. 2008;4(2):297-304. doi:10.2147/vhrm.s993
48. Liberato MV, Nascimento AS, Ayers SD, Lin JZ, Cvoro A, Silveira RL, et al. Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor (PPAR) γ Activators and Pan-PPAR Partial Agonists. PLoS One. 2012;7(5):e36297. doi:10.1371/journal.pone.0036297
49. Agarwal P, Gupta R. Alpha-amylase inhibition can treat diabetes mellitus. Res Rev J Med Health Sci. 2016;5(4):1-8.
50. Deacon CF. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front Endocrinol. 2019;10:80. doi:10.3389/fendo.2019.00080
51. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409-39. doi:10.1152/physrev.00034.2006
52. Kim NH, Kim NH. Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics. Diabetes Metab J. 2022;46(4):543-51. doi:10.4093/dmj.2022.0209
53. Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-9. doi:10.1097/med.0000000000000311
54. Alssema M, Ruijgrok C, Blaak EE, Egli L, Dussort P, Vinoy S, et al. Effects of alpha-glucosidase-inhibiting drugs on acute postprandial glucose and insulin responses: a systematic review and meta-analysis. Nutr Diabetes. 2021;11(1):11. doi:10.1038/s41387-021-00152-5
55. van Breemen RB, Li Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol. 2005;1(2):175-85. doi:10.1517/17425255.1.2.175
56. Alam S, Khan F. Virtual screening, Docking, ADMET and System Pharmacology studies on Garcinia caged Xanthone derivatives for Anticancer activity. Sci Rep. 2018;8:5524. doi:10.1038/s41598-018-23768-7
57. Ertl P, Schuffenhauer A. Estimation of synthetic accessibility score of drug-like molecules based on molecular complexity and fragment contributions. J Cheminform. 2009;1(1):8. doi:10.1186/1758-2946-1-8
58. Potts RO, Guy RH. Predicting skin permeability. Pharm Res. 1992;9(5):663-9. doi:10.1023/a:1015810312465
59. Daina A, Zoete V. A boiled‐egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11(11):1117-21. doi:10.1002/cmdc.201600182
60. Testa B, Kraemer SD. The biochemistry of drug metabolism–an introduction: part 3. Reactions of hydrolysis and their enzymes. Chem Biodivers. 2007;4(9):2031-122. doi:10.1002/cbdv.200790169
61. Saeidnia S, Manayi A, Abdollahi M. The pros and cons of the in-silico pharmaco-toxicology in drug discovery and development. Int J Pharmacol. 2013;9(3):176-81. doi:10.3923/ijp.2013.176.181
62. Drwal MN, Banerjee P, Dunkel M, Wettig MR, Preissner R. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014;42(W1):W53-8. doi:10.1093/nar/gku401
Authors
Copyright (c) 2023 Mubarak Muhammad Dahiru, Neksumi Musa, AbdulAzeez Mumsiri Abaka, Maimuna Abdulrahman Abubakar

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.