Multiple Sclerosis: Current Knowledge of the Pathology and Use of Monoclonal Antibodies as a Promising Therapy
Abstract
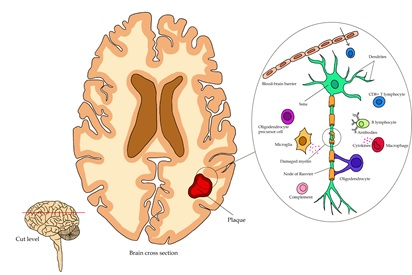
Multiple sclerosis is an autoimmune condition characterized by an inflammatory condition and neuron demyelination, leading to a significant deterioration in the patient's quality of life as the disease progresses. The immune system reactivity in this pathology is mainly mediated by reactive T lymphocytes against myelin. The harmful substances production and proinflammatory cell infiltration occur. Currently, there is no cure, so treatment focuses on reducing the development of the individual's long-term disability by addressing symptoms, acute exacerbations, and slowing progress. The traditional treatment includes immunosuppressive substances such as corticosteroids and interferons. However, an approach to more specific, highly effective therapies such as monoclonal antibodies is currently being sought. Ofatumumab, ocrelizumab, alemtuzumab, and rituximab are commercialized monoclonal antibodies. Likewise, therapies in the research phase, such as ublituximab, inebilizumab, GNbAC1, and elezanumab, can be found. Therefore, research must continue to have more information to increase the availability of therapeutic options for patients.
Full text article
References
2. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-85. doi:10.1016/s1474-4422(18)30443-5
3. Cree BAC, Hauser SL. Multiple Sclerosis. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL, editors. Harrison's Principles of Internal Medicine. 21st edition. New York: McGraw Hill; 2022.
4. Bainbridge JL, Miravalle A, Wong PS, Makelky MJ, Rajkovic S. Multiple Sclerosis. In: Dipiro JT, Yee GC, Haines ST, Nolin TD, Ellingrod VL, Posey LM, editors. DiPiro's Pharmacotherapy: A Pathophysiologic Approach. 12th edition. New York: McGraw Hill; 2023.
5. Evangelopoulos ME, Nasiri-Ansari N, Kassi E, Papadopoulou A, Evangelopoulos DS, Moutsatsou P. Methylprednisolone stimulated gene expression (GILZ, MCL-1) and basal cortisol levels in multiple sclerosis patients in relapse are associated with clinical response. Sci Rep. 2021;11(1):19462. doi:10.1038/s41598-021-98868-y
6. Vargas DL, Tyor WR. Update on disease-modifying therapies for multiple sclerosis. J Investig Med. 2017;65(5):883-91. doi:10.1136/jim-2016-000339
7. Bayer V. An Overview of Monoclonal Antibodies. Semin Oncol Nurs. 2019;35(5):150927. doi:10.1016/j.soncn.2019.08.006
8. Hauser SL, Cree BAC. Treatment of Multiple Sclerosis: A Review. Am J Med. 2020;133(12):1380-90.e2. doi:10.1016/j.amjmed.2020.05.049
9. Voge NV, Alvarez E. Monoclonal Antibodies in Multiple Sclerosis: Present and Future. Biomedicines. 2019;7(1):20. doi:10.3390/biomedicines7010020
10. Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325-38. doi:10.1038/nrd3003
11. Boron WF, Boulpaep EL. Medical Physiology: A Cellular and Molecular Approach. 2nd edition. Philadelphia: Elsevier; 2012.
12. Sakka L, Gabrillargues J, Coll G. Anatomy of the Spinal Meninges. Oper Neurosurg. 2016;12(2):168-88. doi:10.1227/NEU.0000000000001048
13. Yang QQ, Zhou JW. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia. 2019;67(6):1017-35. doi:10.1002/glia.23571
14. Barres BA. The mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron. 2008;60(3):430-40. doi:10.1016/j.neuron.2008.10.013
15. Tafti D, Ehsan M, Xixis KL. Multiple Sclerosis. Treasure Island: StatPearls Publishing. 2022.
16. Porras-Bentancourt M, Núñez-Orozco L, Plascencia-Álvarez NI, Quiñones-Aguilar S, Sauri-Suárez S. Esclerosis Múltiple. Rev Med Neuroci. 2007;8(1):57-66.
17. Scolding NJ, Pasquini M, Reingold SC, Cohen JA, International Conference on Cell-Based Therapies for Multiple Sclerosis. Cell-based therapeutic strategies for multiple sclerosis. Brain. 2017;140(11):2776-96. doi:10.1093/brain/awx154
18. Casini G, Yurashevich M, Vanga R, Dash S, Dhib-Jalbut S, Gerhardstein B, et al. Are Periventricular Lesions Specific for Multiple Sclerosis? J Neurol Neurophysiol. 2013;4(2):150. doi:10.4172/2155-9562.1000150
19. Agrawal SM, Williamson J, Sharma R, Kebir H, Patel K, Prat A, et al. Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain. 2013;136(Pt 6):1760-77. doi:10.1093/brain/awt093
20. Lassmann H. Multiple Sclerosis Pathology. Cold Spring Harb Perspect Med. 2018;8(3):a028936. doi:10.1101/cshperspect.a028936
21. Dheen ST, Kaur C, Ling EA. Microglial Activation and its Implications in the Brain Diseases. Curr Med Chem. 2007;14(11):1189-97. doi:10.2174/092986707780597961
22. Martinez-Altarriba MC, Ramos-Campoy O, Luna-Calcaño IM, Arrieta-Antón E. Revisión de la esclerosis múltiple (1). A propósito de un caso. Semergen. 2015;41(5):261-5. doi:10.1016/j.smerg.2014.07.009
23. Delgado V, León A. Protocolo diagnóstico y terapéutico del brote de enfermedad dismielinizante. Medicine - Programa de Formación Médica Continuada Acreditado. 2015;11(77):4651-5. doi:10.1016/j.med.2015.04.006
24. Vanegas AC, Rodas MIZ, Guerrero MAR. Síndrome clínico aislado: abordaje del primer evento desmielinizante. Acta Neurol Colomb. 2019;35(2):64-73. doi:10.22379/24224022236
25. López-Gómez J, Sacristán-Enciso B, Caro-Miró MA, Pascual MRQ. Síndrome clínico aislado: diagnóstico y riesgo de desarrollar esclerosis múltiple clínicamente definida. Neurología. 2021. doi:10.1016/j.nrl.2021.01.011
26. Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020;16(3):137-53. doi:10.1038/s41582-020-0312-z
27. García JAM, Lax MG, Rico IP, Espinosa P, Navarro MLM. Paresthesias: A case series study. J Neurol Sci. 2017;381:373. doi:10.1016/j.jns.2017.08.1062
28. Sáinz-Pelayo M, Albu S, Murillo N, Benito-Penalva J. Actualización sobre mecanismos fisiopatológicos, avances en el diagnóstico y tratamiento. Rev Neurol. 2020;70(12):453-60. doi:10.33588/rn.7012.2019474
29. Akbar U, Ashizawa T. Ataxia. Neurol Clin. 2015;33(1):225-48. doi:10.1016/j.ncl.2014.09.004
30. Regev K, Weiner HL. Immune Dysregulation in Multiple Sclerosis. In: Arnon R, Miller A, editors. Translational Neuroimmunology in Multiple Sclerosis. San Diego: Elsevier; 2016.
31. Margoni M, Preziosa P, Filippi M, Rocca MA. Anti-CD20 therapies for multiple sclerosis: current status and future perspectives. J Neurol. 2022;269(3):1316-34. doi:10.1007/s00415-021-10744-x
32. Piehl F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J Intern Med. 2021;289(6):771-91. doi:10.1111/joim.13215
33. Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 2015;69(1):142-59. doi:10.1111/prd.12083
34. Kak G, Raza M, Tiwari BK. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol Concepts. 2018;9(1):64-79. doi:10.1515/bmc-2018-0007
35. Wootla B, Watzlawik JO, Stavropoulos N, Wittenberg NJ, Dasari H, Abdelrahim MA, et al. Recent Advances in Monoclonal Antibody Therapies for Multiple Sclerosis. Expert Opin Biol Ther. 2016;16(6):827-39. doi:10.1517/14712598.2016.1158809
36. Villar-Fincheira P, Sanhueza-Olivares F, Norambuena-Soto I, Cancino-Arenas N, Hernandez-Vargas F, Troncoso R, et al. Role of Interleukin-6 in Vascular Health and Disease. Front Mol Biosci. 2021;8:641734. doi:10.3389/fmolb.2021.641734
37. Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021;22(5):2719. doi:10.3390/ijms22052719
38. Liu K, Huang A, Nie J, Tan J, Xing S, Qu Y, et al. IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front Immunol. 2021;12:683332. doi:10.3389/fimmu.2021.683332
39. Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. 2014;32:51-82. doi:10.1146/annurev-immunol-032713-120257
40. Howard J, Trevick S, Younger DS. Epidemiology of Multiple Sclerosis. Neurol Clin. 2016;34(4):919-39. doi:10.1016/j.ncl.2016.06.016
41. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816-21. doi:10.1177/1352458520970841
42. Carod-Artal FJ. The epidemiology of multiple sclerosis in the Scottish Highlands: Prevalence, incidence and time to confirmed diagnosis and treatment initiation. Mult Scler Relat Disord. 2021;47:102657. doi:10.1016/j.msard.2020.102657
43. Cromie D, Mullan F, Hinchliff C, Miller M, McVerry F, McCarron MO. Secular trends in disease modifying treatment and expenditure in multiple sclerosis: A longitudinal population study in the north of Ireland. Mult Scler Relat Disord. 2020;45:102444. doi:10.1016/j.msard.2020.102444
44. Forouhari A, Taheri G, Salari M, Moosazadeh M, Etemadifar M. Multiple sclerosis epidemiology in Asia and Oceania; A systematic review and meta-analysis. Mult Scler Relat Disord. 2021;54:103119. doi:10.1016/j.msard.2021.103119
45. Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-40. doi:10.1212/WNL.0000000000007035
46. Gilmour H, Ramage-Morin PL, Wong SL. Multiple sclerosis: Prevalence and impact. Health Rep. 2018;29(1):3-8.
47. Cristiano E, Rojas JI, Romano M, Frider N, Machnicki G, Giunta DH, et al. The epidemiology of multiple sclerosis in Latin America and the Caribbean: a systematic review. Mult Scler. 2013;19(7):844-54. doi:10.1177/1352458512462918
48. Correa E, Paredes V, Martínez B. Prevalence of multiple sclerosis in Latin America and its relationship with European migration. Mult Scler J Exp Transl Clin. 2016;2:2055217316666407. doi:10.1177/2055217316666407
49. Vásquez-Céspedes J, Fernández-Morales H, Valverde-Espinoza JA, Moraga-López A, Carazo-Céspedes K. Perfil demográfico y clínico de la esclerosis múltiple en Costa Rica: revisión de la casuística nacional a diciembre de 2017. Neurol Argent. 2021;13(2):69-77. doi:10.1016/j.neuarg.2021.02.002
50. Ömerhoca S, Akkaş SY, İçen NK. Multiple Sclerosis: Diagnosis and Differential Diagnosis. Noro Psikiyatr Ars. 2018;55(Suppl 1):S1-9. doi:10.29399/npa.23418
51. Vázquez-Gómez LA, Hidalgo-Mesa C, Beltrán-González BM, Broche-Pérez Y, Mederos-Herrera AM. Perfil epidemiológico, clínico, e imagenológico de la esclerosis múltiple. Medisur. 2021;19(6):948-58.
52. Stern SDC. Multiple Sclerosis. In: Stern SDC, Cifu AS, Altkorn D, editors. Symptom to Diagnosis: An Evidence-Based Guide. 4th edition. New York: McGraw Hill; 2020.
53. Zhang D. Values of magnetic Resonance imaging and Cerebrospinal fluid analysis in the diagnosis of Central Nervous System associated infectious diseases. Pak J Med Sci. 2017;33(5):1065-9. doi:10.12669/pjms.335.13083
54. Mahmood F, Nielsen UG, Jørgensen CB, Brink C, Thomsen HS, Hansen RH. Safety of gadolinium based contrast agents in magnetic resonance imaging-guided radiotherapy - An investigation of chelate stability using relaxometry. Phys Imaging Radiat Oncol. 2022;21:96-100. doi:10.1016/j.phro.2022.02.015
55. Sasso BL, Agnello L, Bivona G, Bellia C, Ciaccio M. Cerebrospinal Fluid Analysis in Multiple Sclerosis Diagnosis: An Update. Medicina. 2019;55(6):245. doi:10.3390/medicina55060245
56. Pachner AR, Li L, Narayan K. Intrathecal antibody production in an animal model of multiple sclerosis. J Neuroimmunol. 2007;185(1-2):57-63. doi:10.1016/j.jneuroim.2007.01.017
57. Gasperi C, Salmen A, Antony G, Bayas A, Heesen C, Kümpfel T, et al. Association of Intrathecal Immunoglobulin G Synthesis With Disability Worsening in Multiple Sclerosis. JAMA Neurol. 2019;76(7):841-9. doi:10.1001/jamaneurol.2019.0905
58. Alhajj M, Farhana A. Enzyme Linked Immunosorbent Assay. Treasure Island: StatPearls Publishing; 2023.
59. Calugaru L, Calugaru GT, Calugaru OM. Evoked Potentials in Multiple Sclerosis Diagnosis and Management. Curr Health Sci J. 2016;42(4):385-9. doi:10.12865/CHSJ.42.04.08
60. Guzmán SA, Cerdá JLR, Agulló EM. Las pruebas funcionales urodinámicas en el diagnóstico de la vejiga neurógena. Rehabilitación. 2005;39(6):343-57. doi:10.1016/S0048-7120(05)74368-1
61. Torad H, Shalaby N, Hussein HA, Sadek SZ, Abdelazim MS, Yehia A, et al. Bladder and urodynamic changes in multiple sclerosis. Egypt J Neurol Psychiatry Neurosurg. 2020;56:47. doi:10.1186/s41983-020-00178-z
62. Oreja-Guevara C, Blanco TA, Ruiz LB, Pérez MÁH, Meca-Lallana V, Ramió-Torrentà L. Cognitive Dysfunctions and Assessments in Multiple Sclerosis. Front Neurol. 2019;10:581. doi:10.3389/fneur.2019.00581
63. Ruet A, Brochet B. Cognitive assessment in patients with multiple sclerosis: From neuropsychological batteries to ecological tools. Ann Phys Rehabil Med. 2020;63(2):154-8. doi:10.1016/j.rehab.2018.01.006
64. Tyshkov C, Pawate S, Bradshaw MJ, Kimbrough DJ, Chitnis T, Gelfand JM, et al. Multiple sclerosis and sarcoidosis: A case for coexistence. Neurol Clin Pract. 2019;9(3):218-27. doi:10.1212/CPJ.0000000000000629
65. Jewells VL, Latchaw RE. CNS Vasculitis-An Overview of This Multiple Sclerosis Mimic: Clinical and MRI Implications. Semin Ultrasound CT MR. 2020;41(3):296-308. doi:10.1053/j.sult.2020.02.004
66. Naeem SB, Niazi F, Baig A, Sadiq H, Sattar M. Primary CNS Lymphoma vs. Tumefactive Multiple Sclerosis: A Diagnostic Challenge. J Coll Physicians Surg Pak. 2018;28(1):66-8. doi:10.29271/jcpsp.2018.01.66
67. Kotulska-Jóźwiak K, Pacheva I, Patrova A, Jurkiewicz EJ, Ivanov I, Kuczyński D, et al. Neuroborreliosis (Lyme Disease) or Multiple Sclerosis? Two cases with overlapping features. J Int Child Neurol Assoc. 2019;1(1):12. doi:10.17724/jicna.2018.112
68. Shah P. Symptomatic management in multiple sclerosis. Ann Indian Acad Neurol. 2015;18(Suppl 1):S35-S42. doi:10.4103/0972-2327.164827
69. Samkoff LM, Goodman AD. Symptomatic Management in Multiple Sclerosis. Neurol Clin. 2011;29(2):449-63. doi:10.1016/j.ncl.2011.01.008
70. Tobin WO. Management of Multiple Sclerosis Symptoms and Comorbidities. Continuum. 2019;25(3):753-72. doi:10.1212/CON.0000000000000732
71. Smets I, Van Deun L, Bohyn C, van Pesch V, Vanopdenbosch L, Dive D, et al. Corticosteroids in the management of acute multiple sclerosis exacerbations. Acta Neurol Belg. 2017;117(3):623-33. doi:10.1007/s13760-017-0772-0
72. Fischer HJ, Finck TLK, Pellkofer HL, Reichardt HM, Lühder F. Glucocorticoid Therapy of Multiple Sclerosis Patients Induces Anti-inflammatory Polarization and Increased Chemotaxis of Monocytes. Front Immunol. 2019;10:1200. doi:10.3389/fimmu.2019.01200
73. Goodin DS. Glucocorticoid treatment of multiple sclerosis. Handb Clin Neurol. 2014;122:455-64. doi:10.1016/B978-0-444-52001-2.00020-0
74. Hojati Z, Kai M, Dehghanian F. Mechanism of Action of Interferon Beta in Treatment of Multiple Sclerosis. In: Minagar A, editor. Multiple Sclerosis: A Mechanistic View. San Diego: Elsevier; 2016.
75. Jakimovski D, Kolb C, Ramanathan M, Zivadinov R, Weinstock-Guttman B. Interferon β for Multiple Sclerosis. Cold Spring Harb Perspect Med. 2018 Nov;8(11):a032003. doi:10.1101/cshperspect.a032003
76. Filipi M, Jack S. Interferons in the Treatment of Multiple Sclerosis: A Clinical Efficacy, Safety, and Tolerability Update. Int J MS Care. 2020;22(4):165-72. doi:10.7224/1537-2073.2018-063
77. Comi G, Amato MP, Bertolotto A, Centonze D, De Stefano N, Farina C, et al. The heritage of glatiramer acetate and its use in multiple sclerosis. Mult Scler Demyelinating Disord. 2016;1:6. doi:10.1186/s40893-016-0010-2
78. Prod'homme T, Zamvil SS. The Evolving Mechanisms of Action of Glatiramer Acetate. Cold Spring Harb Perspect Med. 2019;9(2):a029249. doi:10.1101/cshperspect.a029249
79. La Mantia L, Di Pietrantonj C, Rovaris M, Rigon G, Frau S, Berardo F, et al. Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2016;11(11):CD009333. doi:10.1002/14651858.CD009333.pub3
80. Bazúa-Valenti S, García-Sáinz JA. La esfingosina 1-fosfato y su receptor S1P1: reguladores de la respuesta inmune. Rev Fac Med UNAM. 2012;55(6):53-7.
81. McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021;398(10306):1184-94. doi:10.1016/S0140-6736(21)00244-0
82. Brana C, Frossard MJ, Pescini Gobert R, Martinier N, Boschert U, Seabrook TJ. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 and 5 in human multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2014;40(5):564-78. doi:10.1111/nan.12048
83. Ayzenberg I, Hoepner R, Kleiter I. Fingolimod for multiple sclerosis and emerging indications: appropriate patient selection, safety precautions, and special considerations. Ther Clin Risk Manag. 2016;12:261-72. doi:10.2147/TCRM.S65558
84. Hunter SF, Bowen JD, Reder AT. The Direct Effects of Fingolimod in the Central Nervous System: Implications for Relapsing Multiple Sclerosis. CNS Drugs. 2016;30(2):135-47. doi:10.1007/s40263-015-0297-0
85. Behrangi N, Fischbach F, Kipp M. Mechanism of Siponimod: Anti-Inflammatory and Neuroprotective Mode of Action. Cells. 2019;8(1):24. doi:10.3390/cells8010024
86. Dörr J, Paul F. The Transition From First-Line to Second-Line Therapy in Multiple Sclerosis. Curr Treat Options Neurol. 2015;17(6):354. doi:10.1007/s11940-015-0354-5
87. Upadhayay S, Mehan S. Targeting Nrf2/HO-1 anti-oxidant signaling pathway in the progression of multiple sclerosis and influences on neurological dysfunctions. Brain Disord. 2021;3:100019. doi:10.1016/j.dscb.2021.100019
88. Battino M, Giampieri F, Pistollato F, Sureda A, de Oliveira MR, Pittalà V, et al. Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnol Adv. 2018;36(2):358-70. doi:10.1016/j.biotechadv.2017.12.012
89. Ryan DG, Knatko EV, Casey AM, Hukelmann JL, Dayalan Naidu S, Brenes AJ, et al. Nrf2 activation reprograms macrophage intermediary metabolism and suppresses the type I interferon response. iScience. 2022;25(2):103827. doi:10.1016/j.isci.2022.103827
90. Baker D, Pryce G, Herrod SS, Schmierer K. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult Scler Relat Disord. 2019;30:176-86. doi:10.1016/j.msard.2019.02.018
91. Deeks ED. Cladribine Tablets: A Review in Relapsing MS. CNS Drugs. 2018;32(8):785-96. doi:10.1007/s40263-018-0562-0
92. Alkan SS. Monoclonal antibodies: the story of a discovery that revolutionized science and medicine. Nat Rev Immunol. 2004;4(2):153-6. doi:10.1038/nri1265
93. Buss NAPS, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12(5):615-22. doi:10.1016/j.coph.2012.08.001
94. Ledsgaard L, Ljungars A, Rimbault C, Sørensen CV, Tulika T, Wade J, et al. Advances in antibody phage display technology. Drug Discov Today. 2022;27(8):2151-69. doi:10.1016/j.drudis.2022.05.002
95. Merino AG. Anticuerpos monoclonales. Aspectos básicos. Neurología. 2011;26(5):301-6. doi:10.1016/j.nrl.2010.10.005
96. Viana IMO, Roussel S, Defrêne J, Lima EM, Barabé F, Bertrand N. Innate and adaptive immune responses toward nanomedicines. Acta Pharm Sin B. 2021;11(4):852-70. doi:10.1016/j.apsb.2021.02.022
97. van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front Immunol. 2019;10:548. doi:10.3389/fimmu.2019.00548
98. Pedrioli A, Oxenius A. Single B cell technologies for monoclonal antibody discovery. Trends Immunol. 2021;42(12):1143-58. doi:10.1016/j.it.2021.10.008
99. Brezski RJ, Georgiou G. Immunoglobulin isotype knowledge and application to Fc engineering. Curr Opin Immunol. 2016;40:62-9. doi:10.1016/j.coi.2016.03.002
100. Chapman K, Pullen N, Graham M, Ragan I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nat Rev Drug Discov. 2007;6(2):120-6. doi:10.1038/nrd2242
101. Parray HA, Shukla S, Samal S, Shrivastava T, Ahmed S, Sharma C, et al. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int Immunopharmacol. 2020;85:106639. doi:10.1016/j.intimp.2020.106639
102. Kumar R, Parray HA, Shrivastava T, Sinha S, Luthra K. Phage display antibody libraries: A robust approach for generation of recombinant human monoclonal antibodies. Int J Biol Macromol. 2019;135:907-18. doi:10.1016/j.ijbiomac.2019.06.006
103. Elsbernd PM, Carter JL. Using Monoclonal Antibody Therapies for Multiple Sclerosis: A Review. Biologics. 2021;15:255-63. doi:10.2147/BTT.S267273
104. Rommer PS, Dudesek A, Stüve O, Zettl UK. Monoclonal antibodies in treatment of multiple sclerosis. Clin Exp Immunol. 2014;175(3):373-84. doi:10.1111/cei.12197
105. Florou D, Katsara M, Feehan J, Dardiotis E, Apostolopoulos V. Anti-CD20 Agents for Multiple Sclerosis: Spotlight on Ocrelizumab and Ofatumumab. Brain Sci. 2020;10(10):758. doi:10.3390/brainsci10100758
106. Kang C, Blair HA. Ofatumumab: A Review in Relapsing Forms of Multiple Sclerosis. Drugs. 2022;82(1):55-62. doi:10.1007/s40265-021-01650-7
107. Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N Engl J Med. 2020;383(6):546-57. doi:10.1056/NEJMoa1917246
108. Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, Meuth SG, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28(10):1576-90. doi:10.1177/13524585221079731
109. Cotchett KR, Dittel BN, Obeidat AZ. Comparison of the Efficacy and Safety of Anti-CD20 B Cells Depleting Drugs in Multiple Sclerosis. Mult Scler Relat Disord. 2021;49:102787. doi:10.1016/j.msard.2021.102787
110. Lamb YN. Ocrelizumab: A Review in Multiple Sclerosis. Drugs. 2022;82(3):323-34. doi:10.1007/s40265-022-01672-9
111. Syed YY. Ocrelizumab: A Review in Multiple Sclerosis. CNS Drugs. 2018;32(9):883-90. doi:10.1007/s40263-018-0568-7
112. Gelfand JM, Cree BAC, Hauser SL. Ocrelizumab and Other CD20+ B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics. 2017;14(4):835-41. doi:10.1007/s13311-017-0557-4
113. European Medicines Agency. Summary of Risk Management Plan for Ocrevus (Ocrelizumab). Amsterdam: European Medicines Agency; 2023.
114. Ciardi MR, Iannetta M, Zingaropoli MA, Salpini R, Aragri M, Annecca R, et al. Reactivation of Hepatitis B Virus With Immune-Escape Mutations After Ocrelizumab Treatment for Multiple Sclerosis. Open Forum Infect Dis. 2018;6(1):ofy356. doi:10.1093/ofid/ofy356
115. Mancinelli CR, De Rossi N, Capra R. Ocrelizumab for the Treatment of Multiple Sclerosis: Safety, Efficacy, and Pharmacology. Ther Clin Risk Manag. 2021;17:765-76. doi:10.2147/TCRM.S282390
116. Gross RH, Krieger S. Alemtuzumab in multiple sclerosis: an update. Neurodegener Dis Manag. 2015;5(3):225-32. doi:10.2217/nmt.15.14
117. Gallo P, Centonze D, Marrosu MG. Alemtuzumab for multiple sclerosis: the new concept of immunomodulation. Mult Scler Demyelinating Disord. 2017;2:7. doi:10.1186/s40893-017-0024-4
118. Willis MD, Harding KE, Pickersgill TP, Wardle M, Pearson OR, Scolding NJ, et al. Alemtuzumab for multiple sclerosis: Long term follow-up in a multi-centre cohort. Mult Scler. 2016;22(9):1215-23. doi:10.1177/1352458515614092
119. Havrdova E, Horakova D, Kovarova I. Alemtuzumab in the treatment of multiple sclerosis: key clinical trial results and considerations for use. Ther Adv Neurol Disord. 2015;8(1):31-45. doi:10.1177/1756285614563522
120. Killestein J, van Oosten B. Emerging safety issues in alemtuzumab-treated MS patients. Mult Scler. 2019;25(9):1206-8. doi:10.1177/1352458519851219
121. Lenihan DJ, Alencar AJ, Yang D, Kurzrock R, Keating MJ, Duvic M. Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sézary syndrome. Blood. 2004;104(3):655-8. doi:10.1182/blood-2003-07-2345
122. Brandstadter R, Sand IK. The use of natalizumab for multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:1691-1702. doi:10.2147/NDT.S114636
123. Kotra LP, Park. Therapeutic Approaches to MS and Other Neurodegenerative Diseases. In: Chackalamannil S, Rotella D, Ward SE, editors. Chemistry, Molecular Sciences and Chemical Engineering. Oxford: Elsevier; 2017.
124. Horga A, Tintoré M. Natalizumab for relapsing-remitting multiple sclerosis. Neurología. 2011;26(6):357-68. doi:10.1016/j.nrl.2010.10.004
125. Cohan SL, Lucassen EB, Romba MC, Linch SN. Daclizumab: Mechanisms of Action, Therapeutic Efficacy, Adverse Events and Its Uncovering the Potential Role of Innate Immune System Recruitment as a Treatment Strategy for Relapsing Multiple Sclerosis. Biomedicines. 2019;7(1):18. doi:10.3390/biomedicines7010018
126. Rommer PS, Berger K, Ellenberger D, Fneish F, Simbrich A, Stahmann A, et al. Management of MS Patients Treated With Daclizumab - a Case Series of 267 Patients. Front Neurol. 2020;11:996. doi:10.3389/fneur.2020.00996
127. Bielekova B. Daclizumab Therapy for Multiple Sclerosis. Cold Spring Harb Perspect Med. 2019;9(5):a034470. doi:10.1007/s13311-012-0147-4
128. Baldassari LE, Rose JW. Daclizumab: Development, Clinical Trials, and Practical Aspects of Use in Multiple Sclerosis. Neurotherapeutics. 2017;14(4):842-58. doi:10.1007/s13311-017-0553-8
129. Preiningerova JL, Vachova M. Daclizumab high-yield process in the treatment of relapsing-remitting multiple sclerosis. Ther Adv Neurol Disord. 2017;10(1):67-75. doi:10.1177/1756285616671887
130. Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9884):2167-75. doi:10.1016/S0140-6736(12)62190-4
131. Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell Depletion with Rituximab in Relapsing-Remitting Multiple Sclerosis. N Engl J Med. 2008;358(7):676-88. doi:10.1056/NEJMoa0706383
132. Komori M, Lin YC, Cortese I, Blake A, Ohayon J, Cherup J, et al. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol. 2016;3(3):166-79. doi:10.1002/acn3.293
133. Fox E, Lovett-Racke AE, Gormley M, Liu Y, Petracca M, Cocozza S, et al. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult Scler. 2021;27(3):420-9. doi:10.1177/1352458520918375
134. Steinman L, Fox E, Hartung HP, Alvarez E, Qian P, Wray S, et al. Ublituximab versus Teriflunomide in Relapsing Multiple Sclerosis. N Engl J Med. 2022;387(8):704-14. doi:10.1056/NEJMoa2201904
135. Agius MA, Klodowska-Duda G, Maciejowski M, Potemkowski A, Li J, Patra K, et al. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: Results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler. 2019;25(2):235-45. doi:10.1177/1352458517740641
136. Diebold M, Derfuss T. The monoclonal antibody GNbAC1: targeting human endogenous retroviruses in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419833574. doi:10.1177/1756286419833574
137. Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, et al. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients – A twelve month follow-up. J Neuroimmunol. 2015;285:68-70. doi:10.1016/j.jneuroim.2015.05.019
138. Key B, Lah GJ. Repulsive guidance molecule A (RGMa): a molecule for all seasons. Cell Adh Migr. 2012;6(2):85-90. doi:10.4161/cam.20167
139. Ziemann A, Rosebraugh M, Barger B, Cree B. A Phase 1, Multiple-dose Study of Elezanumab (ABT-555) in Patients with Relapsing Forms of Multiple Sclerosis (S56.001). Neurology. 2019;92(Suppl 15):S56.001. doi:10.1212/WNL.92.15_supplement.S56.001
Authors
Copyright (c) 2023 Josué Castellón-Arias, Luana Gazel-Meléndez, Rebeca Guido-Villalobos, Ariela Jiménez-Díaz, Johana Valera-Rangel, Juan José Mora-Román

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.