Structure Modification of Cinnamic Acid to (E)-1-(3,4-dihydroisoquinoline-2(1H)-yl)-3-phenylprop-2-en-1-one and Antioxidant Activity Test by DPPH Method
Abstract
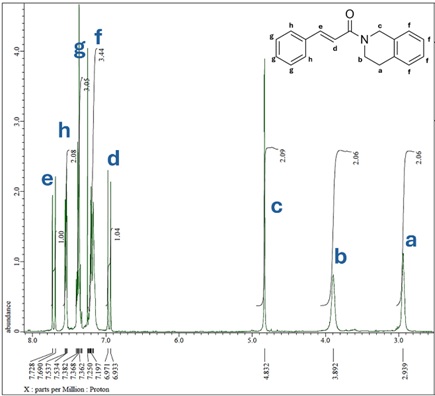
Antioxidants can protect cells from free radical damage by stabilizing them. One of the compounds that has antioxidant activity is cinnamic acid. Cinnamic acid and its derivatives have several activities: antibacterial, anticancer, and antioxidant. However, the ability of cinnamic acid to capture free radicals is still relatively low. One of the efforts that can be made to increase the antioxidant activity of cinnamic acid is to modify its structure. Structure modification is an effort to improve the pharmacological activity of a compound through chemical synthesis reactions. The cinnamic acid structure can be modified by changing the carboxylic -OH group into an amine group through an N-atom acylation reaction. This study was conducted by reacting cinnamoyl chloride (1a), which is a cinnamic acid derivative with 1,2,3,4-tetrahydroisoquinoline (2b) which is a compound of isoquinoline group to produce (E)-1-(3,4-dihydroisoquinoline-2(1H)-yl)-3-phenylprop-2-en-1-one (3b) and then tested for antioxidant activity using DPPH method. The resulting product compound was yellow crystals with a yield of 81.56%. The antioxidant activity produced by the product is more significant than that of cinnamic acid compounds at the same concentration.
Full text article
References
2. Mortada S, Karrouchi K, Hamza EH, Oulmidi A, Bhat MA, Mamad H, et al. Synthesis, structural characterizations, in vitro biological evaluation and computational investigations of pyrazole derivatives as potential antidiabetic and antioxidant agents. Sci Rep. 2024;14(1):1312. DOI: 10.1038/s41598-024-51290-6; PMCID: PMC10789823; PMID: 38225280
3. Sánchez C. Reactive oxygen species and antioxidant properties from mushrooms. Synth Syst Biotechnol. 2016;2(1):13–22. DOI: 10.1016/j.synbio.2016.12.001; PMCID: PMC5625788; PMID: 29062957
4. Hseu YC, Korivi M, Lin FY, Li ML, Lin RW, Wu JJ, et al. Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblasts. J Dermatol Sci. 2018;90(2):123–34. DOI: 10.1016/j.jdermsci.2018.01.004; PMID: 29395579
5. Drakontaeidi A, Pontiki E. Multi-Target-Directed Cinnamic Acid Hybrids Targeting Alzheimer’s Disease. Int J Mol Sci. 2024;25(1):582. DOI: 10.3390/ijms25010582; PMCID: PMC10778916; PMID: 38203753
6. Shollar MM, Merza J, Darwish M, Keshe M. Synthesis, characterization, and biological evaluation of novel cinnamic acid derivatives: cinnamoyl-metronidazole ester and cinnamoyl-memantine amide. Heliyon. 2024;10(9):e29851. DOI: 10.1016/j.heliyon.2024.e29851; PMCID: PMC11058287; PMID: 38694036
7. Natella F, Nardini M, Di Felice M, Scaccini C. Benzoic and cinnamic acid derivatives as antioxidants: Structure- activity relation. J Agric Food Chem. 1999;47(4):1453–9. DOI: 10.1021/jf980737w; PMID: 10563998
8. Pangaribowo DA, Abe M. Photochemical [2 + 2] cycloaddition reaction of carbonyl compounds with Danishefsky diene. Org Biomol Chem. 2020;18(26):4962–70. DOI: 10.1039/d0ob00921k; PMID: 32458869
9. Roussaki M, Zelianaios K, Kavetsou E, Hamilakis S, Hadjipavlou-Litina D, Kontogiorgis C, et al. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorg Med Chem. 2014;22(23):6586–94. DOI: 10.1016/j.bmc.2014.10.008; PMID: 25456384
10. Yang H, Deng M, Jia H, Zhang K, Liu Y, Cheng M, et al. A review of structural modification and biological activities of oleanolic acid. Chin J Nat Med. 2024;22(1):15–30. DOI: 10.1016/s1875-5364(24)60559-5; PMID: 38278556
11. Thangeswaran D, Shamsuddin S, Balakrishnan V. A comprehensive review on the progress and challenges of tetrahydroisoquinoline derivatives as a promising therapeutic agent to treat Alzheimer’s disease. Heliyon. 2024;10(10):e30788. DOI: 10.1016/j.heliyon.2024.e30788; PMCID: PMC11128835; PMID: 38803973
12. Jovanović D, Filipović A, Janjić G, Lazarević-Pašti T, Džambaski Z, Bondžić BP, et al. Targeting Alzheimer’s Disease: Evaluating the Efficacy of C-1 Functionalized N-Aryl-Tetrahydroisoquinolines as Cholinergic Enzyme Inhibitors and Promising Therapeutic Candidates. Int J Mol Sci. 2024;25(2):1033. DOI: 10.3390/ijms25021033; PMCID: PMC10816625; PMID: 38256107
13. AlNeyadi SS, Amer N, Thomas TG, Al Ajeil R, Breitener P, Munawar N. Synthesis, characterization, and antioxidant activity of some 2-methoxyphenols derivatives. Heterocycl Comm. 2020;26(1):112–22. DOI: 10.1515/hc-2020-0112
14. Kariminezhad Z, Rahimi M, Fernandes J, Maltais R, Sancéau JY, Poirier D, et al. Development of New Resolvin D1 Analogues for Osteoarthritis Therapy: Acellular and Computational Approaches to Study Their Antioxidant Activities. Antioxidants. 2024;13(4):386. DOI: 10.3390/antiox13040386; PMCID: PMC11047542; PMID: 38671833
15. Mukhopadhyay N, Ahmed R, Mishra K, Sandbhor R, Sharma RJ, Kaki VR. A validated, precise TLC-densitometry method for simultaneous quantification of mahanimbine and koenimbine in marketed herbal formulations. Futur J Pharm Sci. 2024;10;23. DOI: 10.1186/s43094-024-00591-8
16. Kim HJ, Liu Y, Zeng L. Fourier Transform Infrared (FT-IR) Spectroscopy and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Cotton Fiber Maturity and Crystallinity for Plant Mapping. Sensors. 2024;24(9):2888. DOI: 10.3390/s24092888
17. Kornberger D, Paatsch T, Schmidt M, Salat U. New combined absorption/1H NMR method for qualitative and quantitative analysis of PET degradation products. Environ Sci Pollut Res Int. 2024;31(13):20689–97. DOI: 10.1007/s11356-024-32481-0; PMCID: PMC10927764; PMID: 38393574
18. Yamauchi M, Kitamura Y, Nagano H, Kawatsu J, Gotoh H. DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols. Antioxidants. 2024;13(3):309. DOI: 10.3390/antiox13030309; PMCID: PMC10967577; PMID: 38539842
19. Munawar S, Zahoor AF, Hussain SM, Ahmad S, Mansha A, Parveen B, et al. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon. 2024;10(1):e23416. DOI: 10.1016/j.heliyon.2023.e23416; PMCID: PMC10758822; PMID: 38170008
20. Zhang W, Pinna N. Metal Organic Frameworks Synthesis: The Versatility of Triethylamine. Chemistry. 2024;30(23):e202304256. DOI: 10.1002/chem.202304256; PMID: 38300687
21. Lin Y, Zhou Z, Song Z, Shi Q, Hao Y, Fu Y, et al. Insights into the mechanical stability of tetrahydrofuran hydrates from experimental, machine learning, and molecular dynamics perspectives. Nanoscale. 2024;16(12):6296–308. DOI: 10.1039/d3nr04940j; PMID: 38463012
22. Czopek A, Żmudzki P, Dąbrowska M, Starek M, Łątka K, Bajda M, et al. Reversed-phase thin-layer chromatography and ultra-performance liquid chromatography/mass spectrometry to estimate the drug likeness of phosphodiesterase 10A inhibitors with phthalimide core. JPC-J Planar Chromat. 2024;37:299-308. DOI: 10.1007/s00764-024-00298-9
23. Ambarwati N, Nasution NE. Pemurnian Fraksi Ekstrak Etil Asetat Jamur Endofit Aspergillus salwaensis. Farmasis J Sains Farmasi. 2023;4(1):7-12. DOI: 10.36456/farmasis.v4i1.7006
24. Maryam F, Taebe B, Toding DP. Pengukuran Parameter Spesifik dan Non Spesifik Ekstrak Etanol Daun Matoa (Pometia pinnata J.R & G.Forst). J Mandala Pharmacon Indones. 2020;6(1):1–12. DOI: 10.35311/jmpi.v6i01.39
25. Neto BAD, Beck PS, Sorto JEP, Eberlin MN. In Melting Points We Trust: A Review on the Misguiding Characterization of Multicomponent Reactions Adducts and Intermediates. Molecules. 2022;27(21):7552. DOI: 10.3390/molecules27217552; PMCID: PMC9656178; PMID: 36364380
26. Pavia DL, Lampman GM, Kriz GS. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry. Third Edition. Pacific Grove (CA): Thomson Brooks/Cole; 2009. p. 267–96.
27. Emwas AH, Szczepski K, Poulson BG, Chandra K, McKay RT, Dhahri M, et al. NMR as a "Gold Standard" Method in Drug Design and Discovery. Molecules. 2020;25(20):4597. DOI: 10.3390/molecules25204597; PMCID: PMC7594251; PMID: 33050240
28. Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules. 2022;27(4):1326. DOI: 10.3390/molecules27041326; PMCID: PMC8878429; PMID: 35209118
29. Sayed EM, Hassanien R, Farhan N, Aly HF, Mahmoud K, Bakhite EA. Nitrophenyl-Group-Containing Heterocycles. I. Synthesis, Characterization, Crystal Structure, Anticancer Activity, and Antioxidant Properties of Some New 5,6,7,8-Tetrahydroisoquinolines Bearing 3(4)-Nitrophenyl Group. ACS Omega. 2022;7(10):8767-76. DOI: 10.1021/acsomega.1c06994; PMCID: PMC8928486; PMID: 35309417
Authors
Copyright (c) 2024 Dian Agung Pangaribowo, Fathunnisa Fathunnisa, Ari Satia Nugraha, Ayik Rosita Puspaningtyas, Indah Purnama Sary

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.