Ziziphus rugosa Leaf: Pharmacognostical Characters and Anti-Inflammatory Properties against Carrageenan-Induced Paw Edema
Abstract
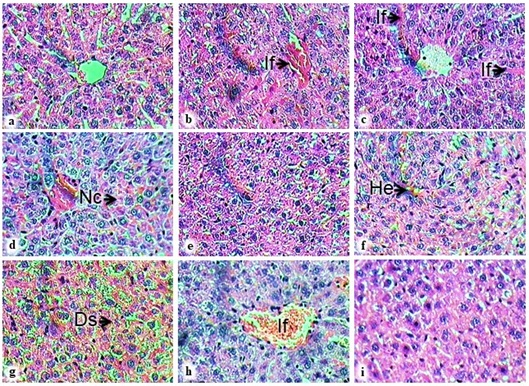
Ziziphus rugosa belongs to the family Rhamnaceae, which includes many flowering species, primarily trees and shrubs, and sometimes vines. This study aims to describe the pharmacognostic characteristics and potential anti-inflammatory properties of Z. rugosa leaf. The pharmacognostical and preliminary phytochemical studies were performed following standard procedures. Acetone, ethanol, and aqueous extracts were screened for anti-inflammatory potential using the carrageenan-induced paw edema model. Ziziphus rugosa was identified by its evergreen nature, recurved hooks, and drupe-type fruits. Leaves are elliptic/rounded with cordate base exhibiting a dark green glossy upper surface and pubescent lower surface. The leaf exhibited a dorsiventral nature in the transverse section, covering trichomes, collenchyma, sclerenchyma patch, and calcium oxalate crystals as key histological characters. Anamocytic stomata, covering trichomes, crystals, and fragments of vessels, are the imperative elements in powder. The extracts contain carbohydrates, alkaloids, glycosides, tannins, saponins, phenolic compounds, proteins, and flavonoids. The acetone extract at 400 and 200 mg/kg displays a maximum inflammation inhibition of 56.96% and 48.77% among the extracts, and the standard diclofenac sodium inhibits inflammation by 65.61% at 24 hours. The altered liver superoxide dismutase, glutathione, and malondialdehyde levels in the positive control group are significantly near normal in the treatment groups. The histopathological studies of treated animals show significant protection against paw and liver tissue damage. Pharmacognostical study outcomes aid in the identification of species along with ascertaining standardization parameters. Further fractionation of acetone extract followed by isolating compounds responsible for the anti-inflammatory activity would provide an alternative to managing inflammation.
Full text article
References
2. Eshete MA, Molla EL. Cultural significance of medicinal plants in healing human ailments among Guji semi-pastoralist people, Suro Barguda District, Ethiopia. J Ethnobiol Ethnomed. 2021;17(1)61. DOI: 10.1186/s13002-021-00487-4; PMCID: PMC8524801; PMID: 34663365
3. Ampomah IG, Malau-Aduli BS, Seidu AA, Malau-Aduli AEO, Emeto TI. Perceptions and Experiences of Orthodox Health Practitioners and Hospital Administrators towards Integrating Traditional Medicine into the Ghanaian Health System. Int J Environ Res Public Health. 2021;18(21):11200. DOI: 10.3390/ijerph182111200; PMCID: PMC8582872; PMID: 34769719
4. Krupa J, Sureshkumar J, Silambarasan R, Priyadarshini K, Ayyanar M. Integration of traditional herbal medicines among the indigenous communities in Thiruvarur District of Tamil Nadu, India. J Ayurveda Integr Med. 2019;10(1):32-7. DOI: 10.1016/j.jaim.2017.07.013; PMCID: PMC6470307; PMID: 30120054
5. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, et al. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules. 2020;25(22):5243. DOI: 10.3390/molecules25225243; PMCID: PMC7697716; PMID: 33187049
6. DeFilipps RA, Krupnick GA. The medicinal plants of Myanmar. PhytoKeys. 2018;102:1-341. DOI: 10.3897/phytokeys.102.24380; PMCID: PMC6033956; PMID: 30002597
7. Manjunatha E, Vedigounder M, Geetha KM, Nandeesh R, Palaksha MN. Review on A Wild Medicinal Plant: Ziziphus rugosa. Int J Pharm Sci Rev Res. 2020;62(2):40-4.
8. Yadav A, Singh P. Analgesic and anti-inflammatory activities of Zizyphus rugosa root barks. J Chem Pharm Res. 2010;2(3):255-9.
9. Mohamad S, Frank RP, Shameem AAK, John NT, Malieka RB. In vivo and in vitro antidiabetic activity of Ziziphus rugosa Lam. Bark. Int J Univers Pharm Bio Sci. 2013;2(5):457-68.
10. Hossain MS, Uddin N, Islam AFMM, Hasan AHMN, Hossain MM, Hasan MR, et al. Evaluation of in vitro antioxidant and brine shrimp lethality activities of different stem extracts of Zizyphus rugosa Lam. J Food Meas Charact. 2015;9(3):454-62. DOI: 10.1007/s11694-015-9253-4
11. Sarala P, Krishnamurthy SR. Phytochemical screening and anthelmintic activity of Zizyphus rugosa Lamk. Int J Pharm Sci Rev Res. 2019;57(1):13-20.
12. Prashith KTR, Raghavendra HL, Vinayaka KS. Evaluation of pericarp and seed extract of Zizyphus rugosa Lam. for cytotoxic activity. Int J Pharm Biol Arch. 2011;2(3):887-90.
13. Prashith KTR, Vinayaka KS, Mallikarjun N, Bharath AC, Shailendra KB, Rakesh KMC, et al. Antibacterial, Insecticidal and Free radical scavenging activity of methanol extract of Ziziphus rugosa Lam. (Rhamnaceae) fruit pericarp. Pharmacogn J. 2011;2(18):65-9. DOI: 10.1016/S0975-3575(11)80028-3
14. Gawande RK, Tare HL, Shende VS, Bongirwar AA, Deore SR, Dama GY. Anxiolytic and CNS depressant activity of extracts obtained from seeds of Ziziphus rugosa. Int J Curr Pharm Clin Res. 2011;1(1):21-32.
15. Bulbul IJ, Khan MF, Rashid MA. Analgesic and central nervous system depressant activities of methanol extract of Ziziphus rugosa Lam. leaves. Afr J Pharm Pharmacol. 2016;10(40):849-53. DOI: 10.5897/AJPP2015.4423
16. Parashar S, Uplanchiwar V, Gautam RK, Goyal S. In vitro antioxidant and in vivo hepatoprotective activity of ethanolic extract of Ziziphus rugosa Lam. Leaves. Indian Drugs. 2019;56(7):69-75. DOI: 10.53879/id.56.07.11577
17. Hossain MS, Uddin N, Hasan N, Hossain MP, Mondal M, Islam T, et al. Phytochemical, cytotoxic, in-vitro antioxidant and anti-microbial investigation of ethanolic leaf extract of Zizyphus rugosa Lam. IOSR J Pharm Biol Sci. 2013;6(5):74-81. DOI: 10.9790/3008-0657481
18. Jain SK, Rao RR. A Hand Book of Field and Herbarium Methods. New Delhi: Today and Tomorrow’s Printers and Publishers; 1976. p. 22–61.
19. Kumar SM, Azamthulla M, Saravanan KS. Pharmacognostical evaluation and anti-convulsant property of Annona reticulata Linn. (Annonaceae) root. Futur J Pharm Sci. 2021;7:173. DOI: 10.1186/s43094-021-00319-y
20. Singh A, Saharan VA, Bhandari A. Pharmacognostic standardization with various plant parts of Desmostachya bipinnata. Pharm Biol. 2014;52(3):298-307. DOI: 10.3109/13880209.2013.834367; PMID: 24107271
21. Kumar V, Sharma AK, Rajput SK, Pal M, Dhiman N. Pharmacognostic and pharmacological evaluation of Eulaliopsis binata plant extracts by measuring in vitro/ in vivo safety profile and anti-microbial potential. Toxicol Res. 2018;7(3):454-64. DOI: 10.1039/c8tx00017d; PMCID: PMC6062097; PMID: 30090595
22. Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. In: Terpenoids. 21st Edition. Pune: Nirali Prakashan; 2017. p. 377-8.
23. Saraf SK, Kumaraswamy V. Basic research: Issues with animal experimentations. Indian J Orthop. 2013;47(1):6-9. DOI: 10.4103/0019-5413.106882; PMCID: PMC3601236; PMID: 23532705
24. Zubaidi SN, Qadi WSM, Maarof S, Misnan NM, Noor HSM, Hamezah HS, et al. Assessing the Acute Toxicological Effects of Annona muricata Leaf Ethanol Extract on Rats: Biochemical, Histopathological, and Metabolomics Analyses. Toxics. 2023;11(8):688. DOI: 10.3390/toxics11080688; PMCID: PMC10458951; PMID: 37624193
25. Ou Z, Zhao J, Zhu L, Huang L, Ma Y, Ma C, et al. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed Pharmacother. 2019;118:109347. DOI: 10.1016/j.biopha.2019.109347; PMID: 31545273
26. Makni S, Tounsi S, Rezgui F, Trigui M, Bouassida KZ. Emex spinosa (L.) Campd. ethyl acetate fractions effects on inflammation and oxidative stress markers in carrageenan induced paw oedema in mice. J Ethnopharmacol. 2019;234:216-24. DOI: 10.1016/j.jep.2018.12.015; PMID: 30552992
27. Haroon HB, Perumalsamy V, Nair G, Anand DK, Kolli R, Monichen J, et al. Repression of polyol pathway activity by Hemidesmus indicus var. pubescens R.Br. Linn root extract, an aldose reductase inhibitor: An in silico and ex vivo study. Nat Prod Bioprospect. 2021;11(3):315–24. DOI: 10.1007/s13659-020-00290-w; PMCID: PMC8141070; PMID: 33284412
28. Li F, Wang Y, Li D, Chen Y, Dou QP. Are we seeing a resurgence in the use of natural products for new drug discovery? Expert Opin Drug Discov. 2019;14(5):417-20. DOI: 10.1080/17460441.2019.1582639; PMID: 30810395
29. Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J Tradit Complement Med. 2017;7(2):234-44. DOI: 10.1016/j.jtcme.2016.05.006; PMCID: PMC5388083; PMID: 28417092
30. Majid N, Nissar S, Raja WY, Nawchoo IA, Bhat ZA. Pharmacognostic standardization of Aralia cachemirica: a comparative study. Futur J Pharm Sci. 2021;7:33. DOI: 10.1186/s43094-021-00181-y
31. Kao D, Henkin JM, Soejarto DD, Kinghorn AD, Oberlies NH. Non-destructive chemical analysis of a Garcinia mangostana L. (Mangosteen) herbarium voucher specimen. Phytochem Lett. 2018;28:124–9. DOI: 10.1016/j.phytol.2018.10.001; PMCID: PMC6317376; PMID: 30613309
32. Noviana E, Indrayanto G, Rohman A. Advances in Fingerprint Analysis for Standardization and Quality Control of Herbal Medicines. Front Pharmacol. 2022;13:853023. DOI: 10.3389/fphar.2022.853023; PMCID: PMC9201489; PMID: 35721184
33. Mboni HM, Faes M, Fraselle S, Compaoré M, Salvius BA, Joseph KB, et al. Evaluating phytochemical constituents and in-vitro antiplasmodial and antioxidant activities of Fadogiella stigmatoloba, Hygrophylla auriculata, Hylodesmum repandum, and Porphyrostemma chevalieri extracts. Heliyon. 2023;9(9):e20103. DOI: 10.1016/j.heliyon.2023.e20103; PMCID: PMC10559859; PMID: 37809863
34. Kong Y, Liu D, Guo X, Chen X. Fluorescence detection of three types of pollutants based on fluorescence resonance energy transfer and its comparison with colorimetric detection. RSC Adv. 2023;13(32):22043-53. DOI: 10.1039/d3ra02647g; PMCID: PMC10359850; PMID: 37483672
35. Campanale C, Savino I, Massarelli C, Uricchio VF. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers. 2023;15(4):911. DOI: 10.3390/polym15040911; PMCID: PMC9961336; PMID: 36850194
36. Pakkirisamy M, Kalakandan SK, Ravichandran K. Phytochemical Screening, GC-MS, FT-IR Analysis of Methanolic Extract of Curcuma caesia Roxb (Black Turmeric). Pharmacogn J. 2017;9(6):952-6. DOI: 10.5530/pj.2017.6.149
37. Erhirhie EO, Ihekwereme CP, Ilodigwe EE. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdiscip Toxicol. 2018;11(1):5-12. DOI: 10.2478/intox-2018-0001; PMCID: PMC6117820; PMID: 30181707
38. Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA. Free Radical-Scavenging, Anti-Inflammatory/Anti-Fibrotic and Hepatoprotective Actions of Taurine and Silymarin against CCl4 Induced Rat Liver Damage. PLoS One. 2015;10(12):e0144509. DOI: 10.1371/journal.pone.0144509; PMCID: PMC4676695; PMID: 26659465
39. Boussouf L, Boutennoune H, Kebieche M, Adjeroud N, Al-Qaoud K, Madani K. Anti-inflammatory, analgesic and antioxidant effects of phenolic compound from Algerian Mentha rotundifolia L. leaves on experimental animals. S Afr J Bot. 2017;113:77-83. DOI: 10.1016/j.sajb.2017.07.003
40. Senthamilselvi MM, Kesavan D, Sulochana N. An anti-inflammatory and anti-microbial flavone glycoside from flowers of Cleome viscosa. Org Med Chem Lett. 2012;2(1):19. DOI: 10.1186/2191-2858-2-19; PMCID: PMC3493290; PMID: 22613049
41. Salem S, Leghouchi E, Soulimani R, Bouayed J. Reduction of paw edema and liver oxidative stress in carrageenan-induced acute inflammation by Lobaria pulmonaria and Parmelia caperata, lichen species, in mice. Int J Vitam Nutr Res. 2021;91(1-2):143-51. DOI: 10.1024/0300-9831/a000620; PMID: 31847731
42. Mansouri MT, Hemmati AA, Naghizadeh B, Mard SA, Rezaie A, Ghorbanzadeh B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J Pharmacol. 2015;47(3):292-8. DOI: 10.4103/0253-7613.157127; PMCID: PMC4450555; PMID: 26069367
Authors
Copyright (c) 2024 Enugurthi Hari Krishna, Kamatchi Sundara Saravanan, Judy Jays

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.