Affinity of Nintedanib Towards New Candidate Target for Idiopathic Pulmonary Fibrosis
Abstract
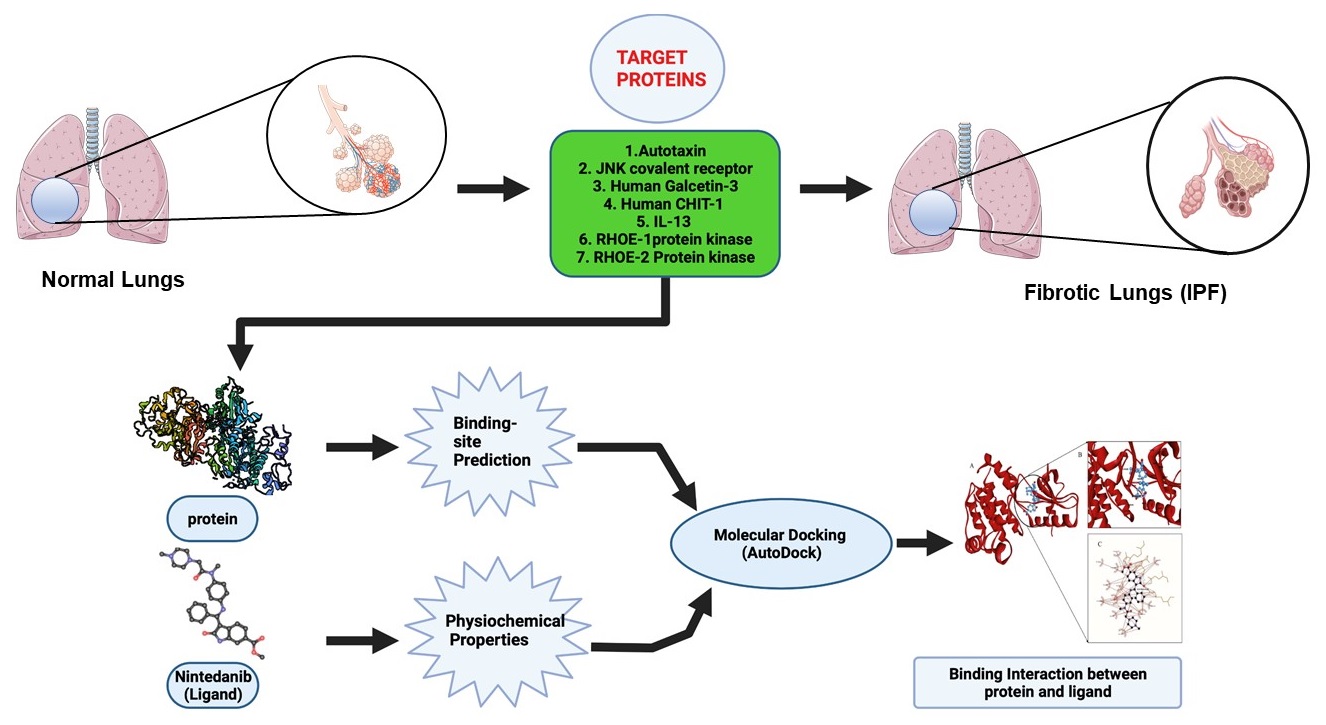
Idiopathic pulmonary fibrosis (IPF) is a progressive disease due to aggregation of fibroblasts on lung parenchyma. Nintedanib, an indolinone-derived tyrosine kinase inhibitor (TKi) has been approved for the treatment of IPF and it is a well-known inhibitor of platelet-derived growth factor (PDGF) receptor-α and -β, fibroblast growth factor (FGF) receptor-1–3 and vascular endothelial growth factor (VEGF) receptor-1–3. This study aims to evaluate the binding interaction between new therapeutic protein candidates for IPF such as autotaxin, galectin-3, interleukin-13, chitotriosidase-1, JNK, RhoE-ROCK-1, ROCK-2 against nintedanib. In this investigation we predicted, computed, and analyzed the binding interactions of the drug nintedanib using an in silico approach called molecular docking. Our docking studies demonstrated that RhoE-ROCK1 and autotaxin showed strong binding affinities towards nintedanib compared to known targets such as VEGFR2 and FGFR1. We can therefore hypothesize a further contribution of nintedanib to the improvement of pathology due to its affinity towards new targets in the pathogenesis of IPF. The next step will be to evaluate the effects of this affinity in vitro on specific cellular models.
Full text article
References
2. Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19(1):32. DOI: 10.1186/s12931-018-0730-2; PMCID: PMC5824456; PMID: 29471816
3. Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primer. 2017;3:17074. DOI: 10.1038/nrdp.2017.74; PMID: 29052582
4. Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345(7):517–25. DOI: 10.1056/nejmra003200; PMID: 11519507
5. Degryse AL, Lawson WE. Progress Toward Improving Animal Models for Idiopathic Pulmonary Fibrosis. Am J Med Sci. 2011;341(6):444–9. DOI: 10.1097/maj.0b013e31821aa000; PMCID: PMC3103078; PMID: 21613932
6. Lancaster LH, de Andrade JA, Zibrak JD, Padilla ML, Albera C, Nathan SD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(146):170057. DOI: 10.1183/16000617.0057-2017; PMCID: PMC9488585; PMID: 29212837
7. Wind S, Schmid U, Freiwald M, Marzin K, Lotz R, Ebner T, et al. Clinical Pharmacokinetics and Pharmacodynamics of Nintedanib. Clin Pharmacokinet. 2019;58(9):1131–47. DOI: 10.1007/s40262-019-00766-0; PMCID: PMC6719436; PMID: 31016670
8. Kolb M, Bonella F, Wollin L. Therapeutic targets in idiopathic pulmonary fibrosis. Respir Med. 2017;131:49–57. DOI: 10.1016/j.rmed.2017.07.062; PMID: 28947042
9. Li R, Jia Y, Kong X, Nie Y, Deng Y, Liu Y. Novel drug delivery systems and disease models for pulmonary fibrosis. J Controlled Release. 2022;348:95–114. DOI: 10.1016/j.jconrel.2022.05.039; PMID: 35636615
10. Hilberg F, Tontsch-Grunt U, Baum A, Le AT, Doebele RC, Lieb S, et al. Triple Angiokinase Inhibitor Nintedanib Directly Inhibits Tumor Cell Growth and Induces Tumor Shrinkage via Blocking Oncogenic Receptor Tyrosine Kinases. J Pharmacol Exp Ther. 2018;364(3):494–503. DOI: 10.1124/jpet.117.244129; PMCID: PMC6040086; PMID: 29263244
11. Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, prodanovich PC, et al. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010;160(7):1699–713. DOI: 10.1111/j.1476-5381.2010.00828.x; PMCID: PMC2936842; PMID: 20649573
12. Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14(1):45–54. DOI: 10.1038/nm1685; PMID: 18066075
13. Stoddard NC, Chun J. Promising pharmacological directions in the world of lysophosphatidic Acid signaling. Biomol Ther. 2015;23(1):1–11. DOI: 10.4062/biomolther.2014.109; PMCID: PMC4286743; PMID: 25593637
14. Cuozzo JW, Clark MA, Keefe AD, Kohlmann A, Mulvihill M, Ni H, et al. Novel Autotaxin Inhibitor for the Treatment of Idiopathic Pulmonary Fibrosis: A Clinical Candidate Discovered Using DNA-Encoded Chemistry. J Med Chem. 2020;63(14):7840–56. DOI: 10.1021/acs.jmedchem.0c00688; PMID: 32584034
15. Hirani N, MacKinnon AC, Nicol L, Ford P, Schambye H, Pedersen A, et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2021;57(5):2002559. DOI: 10.1183/13993003.02559-2020; PMCID: PMC8156151; PMID: 33214209
16. Shimizu Y, Dobashi K, Sano T, Yamada M. Rock Activation in Lung of Idiopathic Pulmonary Fibrosis with Oxidative Stress. Int J Immunopathol Pharmacol. 2014;27(1):37–44. DOI: 10.1177/039463201402700106; PMID: 24674677
17. Knipe RS, Tager AM, Liao JK. The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev. 2015;67(1):103–17. DOI: 10.1124/pr.114.009381; PMCID: PMC4279074; PMID: 25395505
18. Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123(3):1096–108. DOI: 10.1172/jci66700; PMCID: PMC3582144; PMID: 23434591
19. Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779–88. DOI: 10.1172/jci5909; PMCID: PMC408149; PMID: 10079098
20. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018;378(26):2486–96. DOI: 10.1056/nejmoa1804092; PMID: 29782217
21. Panettieri Jr RA, Sjöbring U, Péterffy A, Wessman P, Bowen K, Piper E, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med. 2018;6(7):511–25. DOI: 10.1016/s2213-2600(18)30184-x; PMID: 29792288
22. Hanania NA, Korenblat P, Chapman KR, Bateman ED, Kopecky P, Paggiaro P, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4(10):781–96. DOI: 10.1016/s2213-2600(16)30265-x; PMID: 27616196
23. Maher TM, van der Aar EM, Van de Steen O, Allamassey L, Desrivot J, Dupont S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir Med. 2018;6(8):627–35. DOI: 10.1016/s2213-2600(18)30181-4; PMID: 29792287
24. Di Rosa M, Malaguarnera L. Chitotriosidase: A New Inflammatory Marker in Diabetic Complications. Pathobiology. 2016;83(4):211–9. DOI: 10.1159/000443932; PMID: 27116685
25. Koralewski R, Dymek B, Mazur M, Sklepkiewicz P, Olejniczak S, Czestkowski W, et al. Discovery of OATD-01, a First-in-Class Chitinase Inhibitor as Potential New Therapeutics for Idiopathic Pulmonary Fibrosis. J Med Chem. 2020;63(24):15527–40. DOI: 10.1021/acs.jmedchem.0c01179; PMID: 33078933
26. Sklepkiewicz P, Dymek BA, Mlacki M, Koralewski R, Mazur M, Nejman-Gryz P, et al. Inhibition of CHIT1 as a novel therapeutic approach in idiopathic pulmonary fibrosis. Eur J Pharmacol. 2022;919:174792. DOI: 10.1016/j.ejphar.2022.174792; PMID: 35122869
27. Alcorn JF, van der Velden J, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YMWJ. c-Jun N-Terminal Kinase 1 Is Required for the Development of Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2009;40(4):422–32. DOI: 10.1165/rcmb.2008-0174oc; PMCID: PMC2660560; PMID: 18836136
28. Bennett BL. c-Jun N-terminal kinase-dependent mechanisms in respiratory disease. Eur Respir J. 2006;28(3):651–61. DOI: 10.1183/09031936.06.00012106; PMID: 16946096
29. Lee VY, Schroedl C, Brunelle JK, Buccellato LJ, Akinci OI, Kaneto H, et al. Bleomycin induces alveolar epithelial cell death through JNK-dependent activation of the mitochondrial death pathway. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L521–8. DOI: 10.1152/ajplung.00340.2004; PMID: 16148050
30. Lin CH, Yu MC, Tung WH, Chen TT, et al. Connective tissue growth factor induces collagen I expression in human lung fibroblasts through the Rac1/MLK3/JNK/AP-1 pathway. Biochim Biophys Acta. 2013;1833(12):2823–33. DOI: 10.1016/j.bbamcr.2013.07.016; PMID: 23906792
31. Baroroh U, Muscifa ZS, Destiarani W, Rohmatullah FG, Yusuf M. Molecular interaction analysis and visualization of protein-ligand docking using Biovia Discovery Studio Visualizer. Indones J Comput Biol. 2023;2(1):22-30. DOI: 10.24198/ijcb.v2i1.46322
32. Agu PC, Afiukwa CA, Orji OU, Ezeh EM, Ofoke IH, Ogbu CO, et al. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci Rep. 2023;13(1):13398. DOI: 10.1038/s41598-023-40160-2; PMCID: PMC10435576; PMID: 37592012
33. Yang J, Roy A, Zhang Y. Protein-ligand binding site recognition using complementary binding-specific substructure comparison and sequence profile alignment. Bioinformatics. 2013;29(20):2588-95. DOI: 10.1093/bioinformatics/btt447; PMCID: PMC3789548; PMID: 23975762
34. Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Compout Aided Drug Des. 2011;7(2):146-57. DOI: 10.2174/157340911795677602; PMCID: PMC3151162; PMID: 21534921
35. Fu Y, Zhao J, Chen Z. Insights into the Molecular Mechanisms of Protein-Ligand Interactions by Molecular Docking and Molecular Dynamics Simulation: A Case of Oligopeptide Binding Protein. Comput Math Methods Med. 2018;2018:3502514. DOI: 10.1155/2018/3502514; PMCID: PMC6305025; PMID: 30627209
36. Zhang W, Bell EW, Yin M, Zhang Y. EDock: blind protein-ligand docking by replica-exchange monte carlo simulation. J Cheminform. 2020;12(1):37. DOI: 10.1186/s13321-020-00440-9; PMCID: PMC7251717; PMID: 33430966
Authors
Copyright (c) 2024 Hari Baskar Balasubramanian, Sima Biswas, Maria Talmon, Filippo Patrucco, Piero Emilio Balbo, Luigia Grazia Fresu, Angshuman Bagchi

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.