In Silico Analysis of the Antigastritis Activity of Gedi (Abelmoschus manihot) Flower Flavonoids on H2 Receptor
Abstract
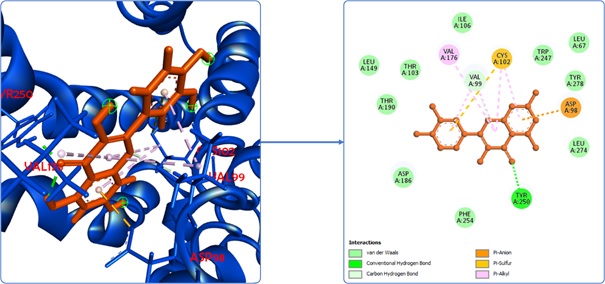
Gastritis remains a highly prevalent health concern in Indonesia, underscoring a continuous demand for innovative therapeutic interventions. The flower of Abelmoschus manihot, commonly known as Gedi, has garnered interest for its potential antigastritis properties, specifically as an H2 antagonist, attributed to its rich flavonoid content. This study aimed to rigorously evaluate the H2 antagonist potential of A. manihot flowers using an in silico approach. Our research methodology involved assessing the physicochemical and pharmacokinetic profiles, alongside molecular docking simulations, of ten prominent flavonoid ligands identified in A. manihot flowers: quercetin, myricetin, myricetin-3-O-glucoside, myricetin-3'-O-glucoside, quercetin-3'-O-glucoside, hibifolin, isoquercetin, hyperoside, quercetin-3-O-robinobioside, and rutin. The analysis of physicochemical and pharmacokinetic properties encompassed Lipinski's Rule of Five and comprehensive ADMET predictions. Molecular docking simulations focused on evaluating binding energies and interactions with crucial H2 receptor residues: Asp98, Asp186, Val99, and Phe254. Among the ligands being assessed, quercetin demonstrated the most favorable physicochemical-pharmacokinetic characteristics and exhibited superior binding affinities and interactions in the molecular docking analysis. These findings collectively suggest that A. manihot flower holds significant promise as a natural source for antigastritis agents, specifically through its potential H2 antagonist activity, with quercetin emerging as a key contributing compound.
Full text article
References
2. Talukder A, Kelly M, Gray D, Sarma H. Prevalence and trends of double burden of malnutrition at household-level in South and Southeast Asia. Discov Public Health. 2024;21:212. DOI: 10.1186/s12982-024-00339-y
3. Mandey JS, Sompie FN, Rustandi, Pontoh CJ. Effects of Gedi Leaves (Abelmoschus manihot (L.) Medik) as a Herbal Plant Rich in Mucilages on Blood Lipid Profiles and Carcass Quality of Broiler Chickens as Functional Food. Proced Food Sci. 2015;3:132-6. DOI: 10.1016/j.profoo.2015.01.013
4. Zhang J, Fu ZL, Chu ZX, Song BW. Gastroprotective Activity of the Total Flavones from Abelmoschus manihot (L.) Medic Flowers. Evid Based Complement Alternat Med. 2020;2020:e6584945. DOI: 10.1155/2020/6584945; PMCID: PMC7060849; PMID: 32184895
5. Yin S, Mei Y, Wei L, Zou L, Cai Z, Wu N, et al. Comparison of Multiple Bioactive Constituents in the Corolla and Other Parts of Abelmoschus manihot. Molecules. 2021;26(7):1864. DOI: 10.3390/molecules26071864; PMCID: PMC8037085; PMID: 33806187
6. Zhang W, Lian Y, Li Q, Sun L, Chen R, Lai X, et al. Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules. 2020;25(20):4626. DOI: 10.3390/molecules25204626; PMCID: PMC7594042; PMID: 33050668
7. Serafim C, Araruna ME, Júnior EA, Diniz M, Hiruma-Lima C, Batista L. A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules. 2020;25(22):5431. DOI: 10.3390/molecules25225431; PMCID: PMC7699562; PMID: 33233494
8. Garg G. Antiulcer Agents. Edited Book of Pharmacology-III [According to Latest Syllabus of B Pharm-VI Semester of Pharmacy Council of India]. Chikkamagaluru: Iterative International Publishers; 2024. DOI: 10.58532/nbennurphch2
9. Tam PK, Saing H. The use of H2-receptor antagonist in the treatment of peptic ulcer disease in children. J Pediatr Gastroenterol Nutr. 1989;8(1):41-6. DOI: 10.1097/00005176-198901000-00009; PMID: 2567346
10. Pathak R, Chandra P. Bioactive Compounds from Myrica esculenta: Antioxidant Insights and Docking Studies on H+K+-ATPase and H2 Receptor Targets. Med Chem. 2025;21. DOI: 10.2174/0115734064366819250125070619; PMID: 39917935
11. Pratama MRF, Poerwono H, Siswodiharjo S. ADMET properties of novel 5-O-benzoylpinostrobin derivatives. J Basic Clin Physiol Pharmacol. 2019;30(6):20190251. DOI: 10.1515/jbcpp-2019-0251; PMID: 31851612
12. Zhang J, Qi T, Wei J. Homology Modeling and Antagonist Binding Site Study of the Human Histamine H2 Receptor. Med Chem. 2012;8(6):1084–92. DOI: 10.2174/1573406411208061084; PMID: 22779803
13. Chagas CM, Moss S, Alisaraie L. Drug metabolites and their effects on the development of adverse reactions: Revisiting Lipinski’s Rule of Five. Int J Pham. 2018;549(1-2):133–49. DOI: 10.1016/j.ijpharm.2018.07.046; PMID: 30040971
14. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3–26. DOI: 10.1016/s0169-409x(00)00129-0; PMID: 11259830
15. Kumari L, Choudhari Y, Patel P, Gupta GD, Singh D, Rosenholm JM, et al. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life. 2023;13(5):1099. DOI: 10.3390/life13051099; PMCID: PMC10221903; PMID: 37240744
16. Arora D, Khurana B. Computer-Aided Biopharmaceutical Characterization: Gastrointestinal Absorption Simulation and In Silico Computational Modeling. In: Saharan VA, editor. Computer Aided Pharmaceutics and Drug Delivery. Singapore: Springer; 2022. DOI: 10.1007/978-981-16-5180-9_7
17. Prachayasittikul V, Prachayasittikul V. P-glycoprotein transporter in drug development. EXCLI J. 2016;15:113–8. DOI: 10.17179/excli2015-768; PMCID: PMC4817426; PMID: 27047321
18. Holt K, Nagar S, Korzekwa K. Methods to Predict Volume of Distribution. Curr Pharmacol Rep. 2019;5(5):391-9. DOI: 10.1007/s40495-019-00186-5; PMCID: PMC8221585; PMID: 34168949
19. del Amo EM, Ghemtio L, Xhaard H, Yliperttula M, Urtti A, Kidron H. Correction: Applying Linear and Non-Linear Methods for Parallel Prediction of Volume of Distribution and Fraction of Unbound Drug. PLoS One. 2015;10(10):e0141943. DOI: 10.1371/journal.pone.0141943; PMCID: PMC4624968; PMID: 26509808
20. Zhao M, Ma J, Li M, Zhang Y, Jiang B, Zhao X, et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int J Mol Sci. 2021;22(23):12808. DOI: 10.3390/ijms222312808; PMCID: PMC8657965; PMID: 34884615
21. Hakkola J, Hukkanen J, Turpeinen M, Pelkonen O. Inhibition and induction of CYP enzymes in humans: an update. Arch Toxicol. 2020;94(11):3671–722. DOI: 10.1007/s00204-020-02936-7; PMCID: PMC7603454; PMID: 33111191
22. Borra SS, Jane NR, Palaniappan D, Subramanian R, Patankar MA, Krishnamoorthy SG, et al. Genetic polymorphism of organic cation transporter 2 (OCT2) and its effects on the pharmacokinetics and pharmacodynamics of Metformin: a narrative review. Egypt J Med Hum Genet. 2023;24:13. DOI: 10.1186/s43042-023-00388-z
23. Iwata H, Matsuo T, Mamada H, Motomura T, Matsushita M, Fujiwara T, et al. Predicting Total Drug Clearance and Volumes of Distribution Using the Machine Learning-Mediated Multimodal Method through the Imputation of Various Nonclinical Data. J Chem Inf Model. 2022;62(17):4057-65. DOI: 10.1021/acs.jcim.2c00318; PMCID: PMC9472274; PMID: 35993595
24. Amorim AMB, Piochi LF, Gaspar AT, Preto AJ, Rosário-Ferreira N, Moreira IS. Advancing Drug Safety in Drug Development: Bridging Computational Predictions for Enhanced Toxicity Prediction. Chem Res Toxicol. 2024;37(6):827-49. DOI: 10.1021/acs.chemrestox.3c00352; PMCID: PMC11187637; PMID: 38758610
25. Bell EW, Zhang Y. DockRMSD: an open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism. J Cheminform. 2019;11(1):40. DOI: 10.1186/s13321-019-0362-7; PMCID: PMC6556049; PMID: 31175455
26. Pratama MRF, Poerwono H, Siswodihardjo S. Molecular docking of novel 5-O-benzoylpinostrobin derivatives as wild type and L858R/T790M/V948R mutant EGFR inhibitor. J Basic Clin Physiol Pharmacol. 2019;30(6):20190301. DOI: 10.1515/jbcpp-2019-0301; PMID: 31855568
27. Kržan M, Keuschler J, Mavri J, Vianello R. Relevance of Hydrogen Bonds for the Histamine H2 Receptor-Ligand Interactions: A Lesson from Deuteration. Biomolecules. 2020;10(2):196. DOI: 10.3390/biom10020196
28. Cao J, Li F, Xia W, Bian W. van der Waals interactions in bimolecular reactions. Chin J Chem Phys. 2019;32(2):157–66. DOI: 10.1063/1674-0068/cjcp1901007
29. Adhav VA, Saikrishnan K. The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function. ACS Omega. 2023;8(25):22268-84. DOI: 10.1021/acsomega.3c00205; PMCID: PMC10308531; PMID: 37396257
30. Panwaria P, Das A. Understanding the n → π* non-covalent interaction using different experimental and theoretical approaches. Phys Chem Chem Phys. 2022;24(37):22371-89. DOI: 10.1039/D2CP02070J
31. Kirsch P, Hartman AM, Hirsch AKH, Empting M. Concepts and Core Principles of Fragment-Based Drug Design. Molecules. 2019;24(23):4309. DOI: 10.3390/molecules24234309; PMCID: PMC6930586; PMID: 31779114
32. Lang PF. Bond order and bond energies. Found Chem. 2024;26:167–77. DOI: 10.1007/s10698-023-09486-7
33. Chourasia M, Cowen T, Friedman-Ezra A, Rubanovich E, Shurki A. The effect of immediate environment on bond strength of different bond types—A valence bond study. J Chem Phys. 2022;157(24):244301. DOI: 10.1063/5.0130020; PMID: 36586970
34. Buchwald P. A Receptor Model With Binding Affinity, Activation Efficacy, and Signal Amplification Parameters for Complex Fractional Response Versus Occupancy Data. Front Pharmacol. 2019;10:605. DOI: 10.3389/fphar.2019.00605; PMCID: PMC6580154; PMID: 31244653
35. Zhao L, Zhi M, Frenking G. The strength of a chemical bond. Int J Quantum Chem. 2022;122(8):e26773. DOI: 10.1002/qua.26773
Authors
Copyright (c) 2025 Gregorius Giani Adikila, Yuanita Amalia Hariyanto, Trina Ekawati Tallei, Elly Juliana Suoth, Sri Sudewi, Fatimawali Fatimawali

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.