Integrative Analysis of the Pharmacological Activities of Lumbricus rubellus: Evidence from Preclinical and Clinical Research
Abstract
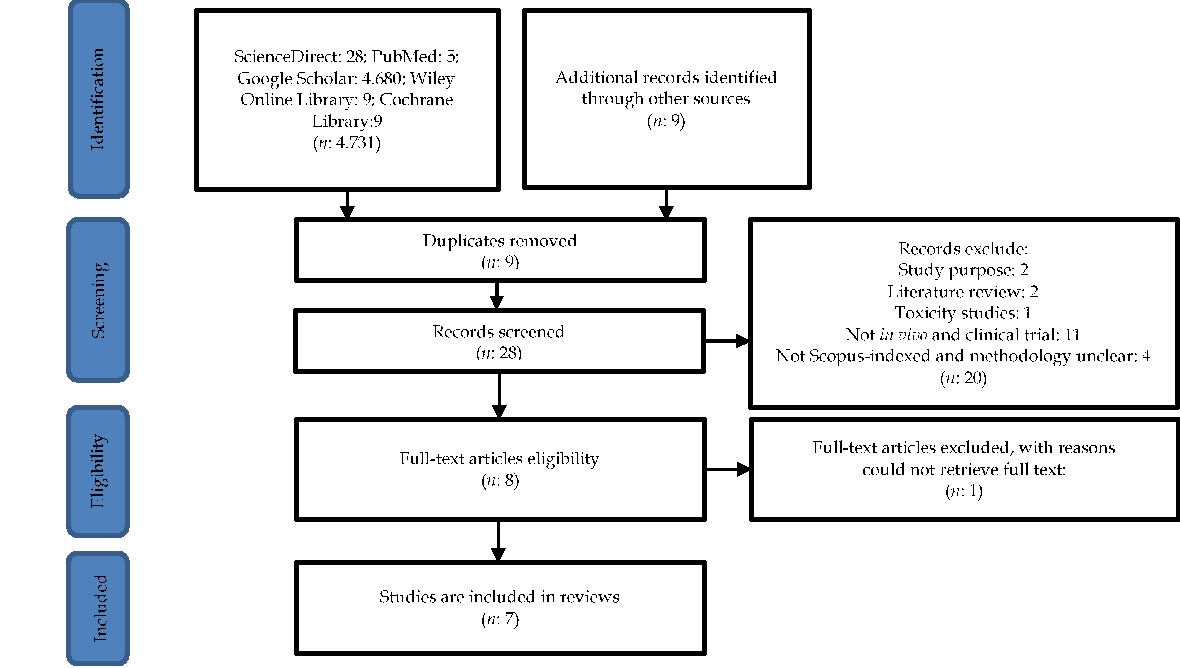
Lumbricus rubellus, commonly known as the red earthworm, has long been used in traditional medicine and contains diverse bioactive compounds with therapeutic potential. However, comprehensive and systematic evaluation of its pharmacological mechanisms remains limited. This review systematically analyzes the pharmacological activities of L. rubellus based on in vivo and clinical trial evidence to provide an integrated scientific understanding of its therapeutic potential. A systematic literature search was performed in PubMed, ScienceDirect, Wiley Online Library, Cochrane Library, and Google Scholar for studies published between 2013 and 2022. The review followed PRISMA 2020 guidelines, applying the PICOS framework for eligibility determination. Study quality was assessed using the ARRIVE checklist for in vivo studies and the JADAD scale for clinical trials. Twelve studies met the inclusion criteria, comprising nine in vivo and three clinical trials. Lumbricus rubellus demonstrated multiple pharmacological effects, including fibrinolytic, antibacterial, hepatoprotective, neuroprotective, and anticancer activities. These effects are mainly attributed to proteins such as lumbrokinase and coelomic fluid metabolites that exhibit antithrombotic, anti-inflammatory, and cytoprotective actions. This review highlights strong evidence supporting the diverse pharmacological activities of L. rubellus and its potential as a natural source for developing novel therapeutic agents. Further standardized clinical investigations are required to confirm its efficacy and safety.
Full text article
References
2. Klein A, Eisenhauer N, Schaefer I. Invasive lumbricid earthworms in North America - different life-histories but common dispersal? J Biogeogr. 2020;47(3):674-85. DOI: 10.1111/jbi.13744; PMCID: PMC7308166; PMID: 32572303
3. Medina-Sauza RM, Álvarez-Jiménez M, Delhal A, Reverchon F, Blouin M, Guerrero-Analco JA, et al. Earthworms building up soil microbiota, a review. Front Environ Sci. 2019;7:81. DOI: 10.3389/fenvs.2019.00081
4. Zhang Q, Liu H, Zhang Y, Ruan H. The complete mitochondrial genome of Lumbricus rubellus (Oligochaeta, Lumbricidae) and its phylogenetic analysis. Mitochondrial DNA B Resour. 2019;4(2):2677–8. DOI: 10.1080/23802359.2019.1644242; PMCID: PMC7706552; PMID: 33365679
5. Venkatachalam S, Chrostyraj JRSS, Don Bosco RB, Yesudhason BV. Antimicrobial peptides from earthworms: Emerging candidates for novel therapeutic applications. Toxicon. 2025;264:108458. DOI: 10.1016/j.toxicon.2025.108458; PMID: 40499792
6. Zhu Z, Deng X, Xie W, Li H, Li Y, Deng Z. Pharmacological effects of bioactive agents in earthworm extract: A comprehensive review. Animal Model Exp Med. 2024;7(5):653-72. DOI: 10.1002/ame2.12465; PMCID: PMC11528390; PMID: 38957072
7. Dharmawati IGAA, Mahadewa TGB, Widyadharma IPE. Antibacterial Activity of Lumbricus Rubellus Earthworm Extract Against Porphyromonas Gingivalis as the Bacterial Cause of Periodontitis. Open Access Maced J Med Sci. 2019;7(6):1032-6. DOI: 10.3889/oamjms.2019.222; PMCID: PMC6454178; PMID: 30976356
8. Foekh NP, Sukrama IDM, Lestari AAW. The ability of earthworm Lumbricus rubellus extract in slowing down the activation of NFkB and TNF-α in lipopolysaccharide-induced Rattus norvegicus. Bali Med J. 2019;8(2):347-52. DOI: 10.15562/bmj.v8i2.1405
9. Sara M, Ilyas F, Hasballah K, Nurjannah N, Harapan H, Mudatsir M. Lumbricus rubellus earthworm as an antibacterial: A systematic review. J Appl Pharm Sci. 2023;13(12):79-86. DOI: 10.7324/JAPS.2023.128228
10. Sara M, Ilyas F, Hasballah K, Nurjannah N, Mudatsir M. The Effects of Lumbricus rubellus Extract on Staphylococcus aureus Colonization and IL-31 Levels in Children with Atopic Dermatitis. Medicina. 2023;59(11):2007. DOI: 10.3390/medicina59112007; PMCID: PMC10672803; PMID: 38004056
11. Ivaldi D, Burgos M, Oltra G, Liquitay CE, Garegnani L. Adherence to PRISMA 2020 statement assessed through the expanded checklist in systematic reviews of interventions: A meta-epidemiological study. Cochrane Evid Synth Methods. 2024;2(5):e12074. DOI: 10.1002/cesm.12074; PMCID: PMC11795886; PMID: 40476264
12. Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020;37(6):387. DOI: 10.1136/emermed-2020-209567; PMID: 32253195
13. Wang L, Hu D, Xu J, Hu J, Wang Y. Complex in vitro Model: A Transformative Model in Drug Development and Precision Medicine. Clin Transl Sci. 2023;17(2):e13695. DOI: 10.1111/cts.13695; PMCID: PMC10828975; PMID: 38062923
14. Su Q, Cheng G, Huang J. A review of research on eligibility criteria for clinical trials. Clin Exp Med. 2023;23(6):1867-79. DOI: 10.1007/s10238-022-00975-1; PMCID: PMC9815064; PMID: 36602707
15. Gusenbauer M, Haddaway NR. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res Synth Methods. 2020;11(2):181-217. DOI: 10.1002/jrsm.1378; PMCID: PMC7079055; PMID: 31614060
16. Patino CM, Ferreira JC. Inclusion and exclusion criteria in research studies: definitions and why they matter. J Bras Pneumol. 2018;44(2):84. DOI: 10.1590/s1806-37562018000000088; PMCID: PMC6044655; PMID: 29791550
17. Veginadu P, Calache H, Gussy M, Pandian A, Masood M. An overview of methodological approaches in systematic reviews. J Evid Based Med. 2022;15(1):39-54. DOI: 10.1111/jebm.12468; PMCID: PMC9322259; PMID: 35416433
18. Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35:49–60. DOI: 10.1007/s10654-019-00576-5; PMID: 31720912
19. Lunny C, Pieper D, Thabet P, Kanji S. Managing overlap of primary study results across systematic reviews: practical considerations for authors of overviews of reviews. BMC Med Res Methodol. 2021;21(1):140. DOI: 10.1186/s12874-021-01269-y; PMCID: PMC8265144; PMID: 34233615
20. Glisic M, Raguindin PF, Gemperli A, Taneri PE, Salvador DJ, Voortman T, et al. A 7-Step Guideline for Qualitative Synthesis and Meta-Analysis of Observational Studies in Health Sciences. Public Health Rev. 2023;44:1605454. DOI: 10.3389/phrs.2023.1605454; PMCID: PMC10227668; PMID: 37260612
21. Moosapour H, Saedifard F, Aalaa M, Soltani A, Larijani B. The rationale behind systematic reviews in clinical medicine: a conceptual framework. J Diabetes Metab Disord. 2021;20(1):919-29. DOI: 10.1007/s40200-021-00773-8; PMCID: PMC8212290; PMID: 34178868
22. Lunny C, Kanji S, Thabet P, Haidich AB, Bougioukas KI, Pieper D. Assessing the methodological quality and risk of bias of systematic reviews: primer for authors of overviews of systematic reviews. BMJ Med. 2024;3(1):e000604. DOI: 10.1136/bmjmed-2023-000604; PMCID: PMC11141200; PMID: 38826514
23. Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20(5):448-52. DOI: 10.1016/s0197-2456(99)00026-4; PMID: 10503804
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. DOI: 10.1136/bmj.n71; PMCID: PMC8005924; PMID: 33782057
25. van Zuuren EJ, Logullo P, Price A, Fedorowicz Z, Hughes EL, Gattrell WT. Existing guidance on reporting of consensus methodology: a systematic review to inform ACCORD guideline development. BMJ Open. 2022;12(9):e065154. DOI: 10.1136/bmjopen-2022-065154; PMCID: PMC9462098; PMID: 36201247
26. Tjandrawinata RR, Trisina J, Rahayu P, Prasetya LA, Hanafiah A, Rachmawati H. Bioactive protein fraction DLBS1033 containing lumbrokinase isolated from Lumbricus rubellus: ex vivo, in vivo, and pharmaceutic studies. Drug Des Devel Ther. 2014; 8:1585-93. DOI: 10.2147/dddt.s66007; PMCID: PMC4181543; PMID: 25284988
27. Trisina J, Sunardi F, Suhartono MT, Tjandrawinata RR. DLBS1033, a protein extract from Lumbricus rubellus, possesses antithrombotic and thrombolytic activities. J Biomed Biotechnol. 2011;2011:519652. DOI: 10.1155/2011/519652; PMCID: PMC3051164; PMID: 21403877
28. Gayatri A, Nafrialdi, Setiabudy RD, Tjandrawinata RR, Susanto LW, Rachman A, et al. A Clinical Trial on Biological Half Life of Bioactive Protein from Lumbricus rubellus, DLBS1033 in Healthy Volunteers. Acta Med Indones. 2018;50(3):208–14.
29. Pinzon RT, Tjandrawinata RR, Wijaya VO, Veronica V. Effect of DLBS1033 on Functional Outcomes for Patients with Acute Ischemic Stroke: A Randomized Controlled Trial. Stroke Res Treat. 2021;2021:5541616. DOI: 10.1155/2021/5541616; PMCID: PMC8049819; PMID: 33927846
30. Pinzon RT, Veronica V. Improvement in Functional Status of Acute Ischemic Stroke Patients Treated with DLBS1033 as Add on Therapy : A Randomized Controlled Study. J Pharm Sci Res. 2020;12(5):667–72.
31. Setyopranoto I, Wibowo S, Tjandrawinata RR. Hemostasis profile and clinical outcome of acute ischemic stroke patients treated with oral lumbrokinase DLBS1033: a comparative study versus aspirin and clopidogrel. Asian J Pharm Clin Res. 2016;9(1):171–7.
32. Lestari AAW, Sukrama IDM, Nurmansyah D. The earthworm (Lumbricus rubellus) extract decreased amino transaminase enzyme level and number of bacterial colony in male wistar rats infected with Salmonella Typhimurium. Biomed Pharmacol J. 2019;12(1):325–32. DOI: 10.13005/bpj/1643
33. Samatra DPGP, Mahadewa Tjokorda GB, Sukrama DM, Dewi NWS, Praja RK, Nurmansyah D, et al. Extract of earthworms (Lumbricus rubellus) reduced malondialdehyde and 8-hydroxy-deoxyguanosine level in male wistar rats infected by salmonella typhi. Biomed Pharmacol J. 2017;10(4):1765–71. DOI: 10.13005/bpj/1290
34. Permana S, Hadi RP, Norahmawati E, Endharti AT. Coelomic fluid of Lumbricus rubellus enhances anti-prolioniferative effect of 5-fluorouracil by modulating focal adhesion kinase express and IL-1β of colorectal cancer in mice. J Appl Pharm Sci. 2019;9(8):41–6. DOI: 10.7324/JAPS.2019.90806
35. Halpern H, Douglas MJ. Jadad scale for reporting randomized controlled trials. In: Halpern H, Douglas MJ, editors. Evidence-based Obstetric Anesthesia. London: Blackwell Publishing; 2005. p. 237–8. DOI: 10.1002/9780470988343.app1
36. Moshina S, Gurushankari B, Niranjan R, Sureshkumar S, Sreenath GS, Kate V. Assessment of the quality of randomized controlled trials in surgery using Jadad score: Where do we stand? J Postgrad Med. 2022;68(4):207-12. DOI: 10.4103/jpgm.jpgm_104_21; PMCID: PMC9841541; PMID: 35417999
37. Minneci PC, Deans KJ. Clinical trials. Semin Pediatr Surg. 2018;27(6):332–7. DOI: 10.1053/j.sempedsurg.2018.10.003; PMID: 30473036
38. Lorian V. Differences between In Vitro and In Vivo Studies. Antimicrob Agents Chemother. 1988;32(10):1600–1. DOI: 10.1128/aac.32.10.1600; PMCID: PMC175930; PMID: 3190189
39. du Sert NP, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Bio. 2020;18(7):e3000411. DOI: 10.1371/journal.pbio.3000411; PMCID: PMC7360025; PMID: 32663221
40. Peters JL, Sutton AJ, Jones DR, Rushton L, Abrams KR. A systematic review of systematic reviews and meta-analyses of animal experiments with guidelines for reporting. J Environ Sci Health B. 2006;41(7):1245-58. DOI: 10.1080/03601230600857130; PMID: 16923604
41. Jalgaonkar S, Mapara T, Verma A, Sayyed M. Comparison of Adherence to ARRIVE Guidelines in Animal Research Articles Published in the Years 2009 and 2016 in Pharmacology Journals: An Observational Study. J Pharmacol Pharmacother. 2019;10(3):77-84. DOI: 10.4103/jpp.JPP_56_19
42. van der Ende NA, Roozenbeek B, Broderick JP, Khatri P, Lingsma HF, Dippel DW. Blinding of outcome assessors and its association with outcome in a randomized open-label stroke trial. Int J Stroke. 2023;18(5):562-8. DOI: 10.1177/17474930221131706; PMCID: PMC10196921; PMID: 36169032
43. Pitre T, Kirsh S, Jassal T, Anderson M, Padoan A, Xiang A, et al. The impact of blinding on trial results: A systematic review and meta-analysis. Cochrane Evid Synth Methods. 2023;1(4):e12015. DOI: 10.1002/cesm.12015; PMCID: PMC11795910; PMID: 40475370
44. Kahan BC, Rehal S, Cro S. Blinded Outcome Assessment Was Infrequently Used and Poorly Reported in Open Trials. PLoS One. 2015;10(6):e0131926. DOI: 10.1371/journal.pone.0131926; PMCID: PMC4488018; PMID: 26120839
45. Mbotwa J, Singini I, Mukaka M. Discrepancy between statistical analysis method and study design in medical research: Examples, implications, and potential solutions. Malawi Med J. 2017;29(1):63-5. DOI: 10.4314/mmj.v29i1.14; PMCID: PMC5442496; PMID: 28567201
46. Villarino NF. Recommendations for a Complete Reporting of Statistical Methods in Veterinary Pharmacology. J Vet Pharmacol Ther. 2025;48(4):221-33. DOI: 10.1111/jvp.70001; PMCID: PMC12257266; PMID: 40474839
47. Festing MFW. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47(1):5-14. DOI: 10.1093/ilar.47.1.5; PMID: 16391426
48. Kousholt BS, Præstegaard KF, Stone JC, Thomsen AF, Johansen TT, Ritskes-Hoitinga M, et al. Reporting of 3Rs Approaches in Preclinical Animal Experimental Studies—A Nationwide Study. Animals. 2023;13(19):3005. DOI: 10.3390/ani13193005
49. Grimm H, Biller-Andorno N, Buch T, Dahlhoff M, Davies G, Cederroth CR, et al. Advancing the 3Rs: innovation, implementation, ethics and society. Front Vet Sci. 2023;10:1185706. DOI: 10.3389/fvets.2023.1185706; PMCID: PMC10310538; PMID: 37396988
50. Strech D, Dirnagl U. 3Rs missing: animal research without scientific value is unethical. BMJ Open Sci. 2019;3(1):bmjos-2018-000048. DOI: 10.1136/bmjos-2018-000048; PMCID: PMC8647585; PMID: 35047678
51. Xu A, Zhang H, Zhang Y, Wu J, Huang Z. Ischemic stroke and intervention strategies based on the timeline of stroke progression: Review and prospects. Acta Pharm Sin B. 2025;15(9):4543-81. DOI: 10.1016/j.apsb.2025.07.026; PMCID: PMC12491693; PMID: 41049732
52. Dorado L, Millán M, Dávalos A. Reperfusion therapies for acute ischemic stroke: an update. Curr Cardiol Rev. 2014;10(4):327-35. DOI: 10.2174/1573403x10666140320144637; PMCID: PMC4101197; PMID: 24646159
53. Wiyarta E, Hidayat R, Kurniawan M, Saputro BIL, Maharani IL, Rampengan DDCH, et al. Therapeutic Potential of Lumbrokinase in Acute Ischemic Stroke: A Meta-Analysis of Efficacy and Safety. Ther Clin Risk Manag. 2025;21:1319-31. DOI: 10.2147/tcrm.s537232; PMCID: PMC12417717; PMID: 40933244
54. Nguyen QTT, Rhee H, Kim M, Lee MY, Lee EJ. Lumbrokinase, a Fibrinolytic Enzyme, Prevents Intra-Abdominal Adhesion by Inhibiting the Migrative and Adhesive Activities of Fibroblast via Attenuation of the AP-1/ICAM-1 Signaling Pathway. Biomed Res Int. 2023;2023:4050730. DOI: 10.1155/2023/4050730; PMCID: PMC9851794; PMID: 36685669
55. Roth JM. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. Proc (Bayl Univ Med Cent). 2011;24(3):257-9. DOI: 10.1080/08998280.2011.11928729; PMCID: PMC3124916; PMID: 21738304
56. Mardiono MML. Peran Lumbrokinase pada Tata Laksana Stroke Iskemik. Cermin Dunia Kedokteran. 2024;51(2):95-8. DOI: 10.55175/cdk.v51i2.1021
57. Nazir A, Nazir A, Zuhair V, Aman S, Sadiq SUR, Hasan AH, et al. The Global Challenge of Antimicrobial Resistance: Mechanisms, Case Studies, and Mitigation Approaches. Health Sci Rep. 2025;8(7):e71077. DOI: 10.1002/hsr2.71077; PMCID: PMC12284435; PMID: 40704322
58. Magfirah, Bengkati SG, Tandi J, Anggi V. Test of the Effectiveness of Earthworm Flour (Lumbricus rubellus) Gastroretentive Mucoadhesive Granule Formulation on Male White Rats (Rattus norvegicus) Infected with Salmonella typhi. J Penelitian Pendidikan IPA. 2024;10(9):6612-20. DOI: 10.29303/jppipa.v10i9.8953
59. Kartikaningsih H, Maharani S, Sartika F. Antibacterial Activity Ethyl Acetate Extracts of Earthworms (Lumbricus rubellus, Eisenia foetida, Nereis sp) Toward Staphylococcus aureus, Enterococcus faecalis, Salmonella thyposa Invitro. El-Hayah J Biol. 2019;7(2):62-73. DOI: 10.18860/elha.v7i2.8248
60. Lestari ES, Severin JA. Antimicrobial Resistance in Indonesia: Prevalence, determinants and genetic basis. Rotterdam: Erasmus University Medical Center; 2009. Handle: 1765/17713
61. Nailufar F, Tjandrawinata RR, Suhartono MT. Thrombus Degradation by Fibrinolytic Enzyme of Stenotrophomonas sp. Originated from Indonesian Soybean-Based Fermented Food on Wistar Rats. Adv Pharmacol Sci. 2016;2016:4206908. DOI: 10.1155/2016/4206908; PMCID: PMC5011239; PMID: 27635131
62. Li GQ, Wang KY, Li DH, Wang N, Liu D. Cloning, expression and characterization of a gene from earthworm Eisenia fetida encoding a blood-clot dissolving protein. PLoS One. 2012;7(12):e53110. DOI: 10.1371/journal.pone.0053110; PMCID: PMC3531398; PMID: 23300872
63. Permana S, Fityanti RP, Norahmawati E, Iskandar A, Mulyadi EDA, Endharti AT. Coelomic Fluid of Eisenia fetida Ameliorates Cetuximab to Reduce K-Ras and Vimentin Expression through Promoting RUNX3 in an AOM/DSS-Induced Colitis Associated Colon Cancer. Evd Based Complement Alternat Med. 2020;2020:9418520. DOI: 10.1155/2020/9418520; PMCID: PMC7387963; PMID: 32765634
64. Endharti AT, Purnamasari Y, Primasari R, Poeranto S, Permana S. Coelomic Fluid of Lumbricus rubellus Synergistically Enhances Cytotoxic Effect of 5-Fluorouracil through Modulation of Focal Adhesion Kinase and p21 in HT-29 Cancer Cell Line. ScientificWorldJournal. 2019:2019:5632859. DOI: 10.1155/2019/5632859; PMCID: PMC6487099; PMID: 31097925
65. El Omari N, El Fessikh M, Aboulaghras S, Bakrim S, Khalid A, Abdalla AN, et al. The role of inflammation in colorectal Cancer and the preventive potential of natural compounds. J Funct Foods. 2025;129:106857. DOI: 10.1016/j.jff.2025.106857
66. Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Sig Transduct Target Ther. 2023;8:198. DOI: 10.1038/s41392-023-01460-1
67. Tan X, Yan Y, Song B, Zhu S, Mei Q, Wu K. Focal adhesion kinase: from biological functions to therapeutic strategies. Exp Hematol Oncol. 2023;12(1):83. DOI: 10.1186/s40164-023-00446-7; PMCID: PMC10519103; PMID: 37749625
68. Vyas D, Laput G, Vyas AK. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014;7:1015-23. DOI: 10.2147/ott.s60114; PMCID: PMC4061164; PMID: 24959088
69. Chandimali N, Bak SG, Park EH, Lim HJ, Won YS, Kim EK, et al. Free radicals and their impact on health and antioxidant defenses: a review. Cell Death Discov. 2025;11:19. DOI: 10.1038/s41420-024-02278-8
70. Zahra KF, Lefter R, Ali A, Abdellah EC, Trus C, Ciobica A, et al. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxid Med Cell Longev. 2021;2021:9965916. DOI: 10.1155/2021/9965916; PMCID: PMC8360750; PMID: 34394838
71. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603-16. DOI: 10.1016/j.freeradbiomed.2010.09.006; PMCID: PMC2990475; PMID: 20840865
Authors
Copyright (c) 2025 Iyan Hardiana, Elly Wahyudin, Muhammad Aswad, Rina Agustina

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.