Method Development and Characterization of Liposomal Formulation of Isotretinoin
Abstract
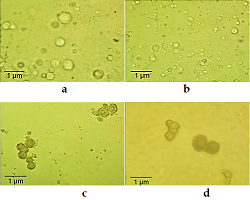
This study aims to develop a liposomal drug delivery system of isotretinoin, an acne drug-using spray drying, as a cost-effective and time-effective technique. The liposomal formulation was prepared by using spray drying; three different strategies were adopted: suspension spray drying (SSD), thin-film hydration and spray drying (TFHSD), and emulsion spray drying (ESD). Isotretinoin was 99% bound with lipid, so lipids hydrogenated soy phosphatidylcholine (HSPC), distearoyl phosphatidylglycerol (DSPG), and cholesterol were selected for the formulation development. The HSPC, DSPG, cholesterol, and isotretinoin were taken in the ratio 4 : 1 : 0.16 : 3.1 mmol. In vitro drug release studies, microscopy, drug content, and related substance characterizations were done to formulate each strategy of spray drying prepared dry liposomes of isotretinoin. Results were compared with the USP monograph of isotretinoin. It was revealed that isotretinoin's liposomal formulation using ESD was having drug release according to the USP limits. Drug content was also according to the USP requirement; no free drug crystals were found in microscopy, multivesicular vesicles were found in shape, a particle size of up 60 µ was found. The ESD technique was a successful, time-effective, and cost-effective technique for preparing a liposomal drug delivery system for isotretinoin.
Full text article
References
2. Mehta PP, Ghoshal D, Pawar AP, Kadam SS, Dhapte-Pawar VS. Recent advances in inhalable liposomes for treatment of pulmonary diseases: Concept to clinical stance. J Drug Deliv Sci Technol. 2020;56(A):101509. doi:10.1016/j.jddst.2020.101509
3. Tan AU, Schlosser BJ, Paller AS. A review of diagnosis and treatment of acne in adult female patients. Int J Womens Dermatol. 2018;4(2):56-71. doi:10.1016/j.ijwd.2017.10.006
4. Rathi SK. Acne Vulgaris Treatment : The Current Scenario. Indian J Dermatol. 2011;56(1):7-13. doi:10.4103/0019-5154.77543
5. Khalil NY, Darwish IA, Al-Qahtani AA. Chapter Five – Isotretinoin. Profiles Drug Subst Excip Relat Methodol. 2020;45:119-57. doi:10.1016/bs.podrm.2019.10.005
6. Tran PT, Berman HS, Leavitt E, Hogeling M, Cheng CE. Analysis of factors associated with relapse in patients on their second course of isotretinoin for acne vulgaris. J Am Acad Dermatol. 2021;84(3):856-9. doi:10.1016/j.jaad.2020.10.030
7. De Holanda PMM, Guilherme K, Barbosa N, Menezes PDL, Nóbrega DF, Dos Santos AF, et al. Salivary Changes Arising from Anti Acne Therapy with Isotretinoin Oral Use: A Systematic Review with Meta-Analyses. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(3):e253. doi:10.1016/j.oooo.2020.04.666
8. Marson JW, Baldwin HE. An Overview of Acne Therapy, Part 2: Hormonal Therapy and Isotretinoin. Dermatol Clin. 2019;37(2):195-203. doi:10.1016/j.det.2018.12.002
9. Sadeghzadeh-Bazargan A, Ghassemi M, Goodarzi A, Roohaninasab M, Nobari NN, Behrangi E. Systematic review of low-dose isotretinoin for treatment ofacne vulgaris: Focus on indication, dosage, regimen, efficacy,safety, satisfaction, and follow up, based on clinical studies. Dermatol Ther. 2021;34:e14438. doi:10.1111/dth.14438
10. Costa CS, Bagatin E, Martimbianco ALC, da Silva EM, Lúcio MM, Magin P, et al. Oral isotretinoin for acne. Cochrane Database Syst Rev. 2018;11(11):CD009435. doi:10.1002/14651858.cd009435.pub2
11. Kuznetsova DA, Gabdrakhmanov DR, Gaynanova GA, Vasileva LA, Kuznetsov DM, Lukashenko SS, et al. Novel biocompatible liposomal formulations for encapsulation of hydrophilic drugs – Chloramphenicol and cisplatin. Colloids Surf A Physicochem Eng Asp. 2021;610:125673. doi:10.1016/j.colsurfa.2020.125673
12. Ansar SM, Mudalige T. Characterization of doxorubicin liposomal formulations for size-based distribution of drug and excipients using asymmetric-flow field-flow fractionation (AF4) and liquid chromatography-mass spectrometry (LC-MS). Int J Pharm. 2020;574:118906. doi:10.1016/j.ijpharm.2019.118906
13. Sonkar R, Sonali, Jha A, Viswanadh MK, Burande AS, Narendra, et al. Gold liposomes for brain-targeted drug delivery: Formulation and brain distribution kinetics. Mater Sci Eng C. 2021;120:111652. doi:10.1016/j.msec.2020.111652
14. Liu W, Hou Y, Jin Y, Wang Y, Xu X, Han J. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends Food Sci Technol. 2020;104:177-89. doi:10.1016/j.tifs.2020.08.012
15. Leitgeb M, Knez Ž, Primožič M. Sustainable technologies for liposome preparation. J Supercrit Fluids. 2020;165:104984. doi:10.1016/j.supflu.2020.104984
16. Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: Advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154-155:102-22. doi:10.1016/j.addr.2020.07.002
17. Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4-22. doi:10.1016/j.addr.2020.06.022
18. Papich MG, Martinez MN. Applying Biopharmaceutical Classification System (BCS) Criteria to Predict Oral Absorption of Drugs in Dogs: Challenges and Pitfalls. AAPS J. 2015;17(4):948-64. doi:10.1208/s12248-015-9743-7
19. Jadhav K, Dhamecha D, Tate A, Tambe H, Patil MB. Application of UV spectrophotometric method for easy and rapid estimation of lafutidine in bulk and pharmaceutical formulation. Pharm Methods. 2011;2(4):264-7. doi:10.4103/2229-4708.93398
20. Singh R, Lillard Jr JW. Nanoparticle-based targeted drug delivery. 2009. Exp Mol Pathol. 86(3):215-23. doi:10.1016/j.yexmp.2008.12.004
21. Durowoju IB, Bhandal KS, Hu J, Carpick B, Kirkitadze M. Differential Scanning Calorimetry — A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J Vis Exp. 2017;121:55262. doi:10.3791/55262
22. Ji W, Li X, Wang J, Ye B, Wang C. Preparation and Characterization of the Solid Spherical HMX/F2602 by the Suspension Spray-Drying Method. J Energ Mater. 2016;34(4):357-67. doi:10.1080/07370652.2015.1095813
23. Varina S, Martin A, Cocero MJ. Liposomal Incorporation of Lavandin Essential Oil by a Thin-Film Hydration Method and by Particles from Gas-Saturated Solutions. Ind Eng Chem Res. 2011;50(4):2088-97. doi:10.1021/ie102016r
24. Zhang H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Methods Mol Biol. 2017;1522:17-22. doi:10.1007/978-1-4939-6591-5_2
25. Maniyar MG, Kokare CR. Formulation and evaluation of spray dried liposomes of lopinavir for topical application. J Pharm Investig. 2019;49:259-70. doi:10.1007/s40005-018-0403-7
26. Juttulapa M, Piriyaprasarth S, Takeuchi H, Sriamornsak P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J Pharm Sci. 2017;12(1):21-7. doi:10.1016/j.ajps.2016.09.004
27. Eroğlu İ, Aslan M, Yaman Ü, Gultekinoglu M, Çalamak S, Kart D, et al. Liposome-based combination therapy for acne treatment. J Liposome Res. 2020;30(3):263-73. doi:10.1080/08982104.2019.1630646
28. Madan S, Nehate C, Barman TK, Rathore AS, Koul V. Design, preparation, and evaluation of liposomal gel formulations for treatment of acne: in vitro and in vivo studies. Drug Dev Ind Pharm. 2019;45(3):395-404. doi:10.1080/03639045.2018.1546310
29. Benne N, Leboux RJT, Glandrup M, van Dujin J, Vigario FL, Neustrup MA, et al. Atomic force microscopy measurements of anionic liposomes reveal the effect of liposomal rigidity on antigen-specific regulatory T cell responses. J Control Release. 2020;318:246-55. doi:10.1016/j.jconrel.2019.12.003
30. Weber F, Rahnfeld L, Luciani P. Analytical profiling and stability evaluation of liposomal drug delivery systems: A rapid UHPLC-CAD-based approach for phospholipids in research and quality control. Talanta. 2020;220:121320. doi:10.1016/j.talanta.2020.121320
31. Franzé S, Selmin F, Samaritani E, Minghetti P, Cilurzo F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging, Pharmaceutics. 2018;10(3):139. doi:10.3390/pharmaceutics10030139
32. Layegh P, Mosallaei N, Bagheri D, Jaafari MR, Golmohammadzadeh S. The efficacy of Isotretinoin-loaded solid lipid nanoparticles in comparison to Isotrex® on acne treatment. Nanomed J. 2013;1(1):38-47.
33. Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: A case series with sixty patients. J Family Med Prim Care. 2018;7(1):171-4. doi:10.4103/jfmpc.jfmpc_104_16
34. Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi:10.1186/1556-276X-8-102
35. Vranić E, Uzunović A. Study of The Applicabilty of Content Uniformity and Dissolution Variation Test on Ropinirole Hydrochloride Tablets. Bosn J Basic Med Sci. 2008;8(2):193-200. doi:10.17305/bjbms.2008.2981
36. Nurhikmah W, Sumirtapura YC, Pamudji JS. Dissolution Profile of Mefenamic Acid Solid Dosage Forms in Two Compendial and Biorelevant (FaSSIF) Media. Sci Pharm. 2016;84(1):181-90. doi:10.3797/scipharm.ISP.2015.09
37. Taylor PW, Keenan MHJ. Pharmaceutical quality of generic isotretinoin products, compared with Roaccutane. Curr Med Res Opin. 2006;22(3):603-15. doi:10.1185/030079906x96326
Authors
Copyright (c) 2021 Md Iftekhar Ahmad, Punet Kumar, Sangam Singh, Nitin Kumar

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.