Chemical Constituents and Antioxidant Activity of Melothria scabra Naudin Fruits
Abstract
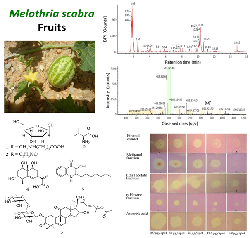
The fruit of Melothria scabra Naudin is traditionally used by natives of South East Sulawesi and has economic values in the local markets. Nonetheless, little scientific information was gained from this plant to support its development for nutraceutical and pharmaceutical aspects. This study aimed to investigate the phytochemicals contained in the ethanol extract and organic fractions (methanol, ethyl acetate, and hexane) of the fruits using specific reagents and an LC-MS/MS analysis, as well as to evaluate their total phenolics, total flavonoids, and DPPH radical scavenging activity using a dot-blot staining and spectrophotometric assays. Results showed that the fruits of M. scabra contained alkaloids, tannins, flavonoids, terpenoids, and saponins. Six compounds were successfully identified from the ethanol extract of the fruits for the first time that is D-1-[(3-carboxypropyl)amino]-1-deoxyfructose (1), fructose-C3H5NO (2), valine (3), 1β, 3α, 9β-trihydroxyeudesma-5,11(13)-dien-12-oic acid (4), Cucurbitacin B-2-O-α-L-rhamnopyranosyl-β-D-glucopyranoside (5), and 2-heptyl-3-hydroxy-4(H)-quinolone (6). Total phenolics in the extract and organic fractions were in the range of 54.2 ± 2.4 to 259.1 ± 8.4 mg GAE/g, while total flavonoids were in the range of 1.6 ± 0.2 to 22.4 ± 0.2 mg QE/g. The ethanol extract and its organic fractions (methanol and ethyl acetate) were potent radical scavengers with SC50 values ranging from 20.7 to 37.5 µg/mL when compared with ascorbic acid, gallic acid, and quercetin (SC50 of 2.8 to 9.4 µg/mL). This study concludes that M. scabra fruits could be developed as a source of natural antioxidant agents for nutraceuticals and pharmaceuticals purposes.
Full text article
References
2. Arniawati, Kahirun, Arafah N, Iryan. Kontribusi kawasan Taman Nasional Rawa Aopa Watumohai sebagai Penyedia Pangan bagi Masyarakat Desa Tatangge. J Eco Green. 2019;5(1):83-8.
3. Ahmed I, Nasir S, Yousaf I, Ahmad B, Zafar S, Javaid A. Plant Extracts along with Selective Chemicals and Bacillus thuringiensis israelensis: A Novel Approach to Tackle the Problem of Insecticidal Resistance in Mosquiotoes. Pak J Agri Sci. 2019;56(4):905-11. doi:10.21162/PAKJAS/19.8481
4. Anusha G, Sunayana R, Ponnam M, Kumar BA. Phytochemical Investigation and In Vitro Antidiabetic Activity of Melothria scabra. Asian J Pharm Res Dev. 2019;7(4):43-48. doi:10.22270/ajprd.v7i4.553
5. Sabandar CW, Jalil J, Ahmat N, Aladdin, N-A, Kamaruddin S, Wahyuningrum R. Aktivitas Antioxidant dan Penghambatan Xantin Oksidase Kulit Batang Songi (Dillenia serrata Thunb.). J Farmasi Galenika. 2020;6(1):151-9. doi:10.22487/j24428744.2020.v6.i1.15008
6. Sahidin I, Sabandar CW, Wahyuni, Hamsidi R, Mardikasari SA, Zubaydah WOS, et al. Investigation of Compounds and Biological Activity of Selected Indonesian Marine Sponges. Nat Prod J. 2019;10(3):312-21. doi:10.2174/2210315509666190627105237
7. Sabandar CW, Jalil J, Ahmat N, Aladdin N-A. Assessment of Antioxidant and Xanthine Oxidase Inhibitory Activity of Triadia conchinchinensis Stem Bark. Curr Res Biosci Biotechnol. 2019;1(1):39-44.
8. Al-Farsi M, Al-Amri A, Al-Hadhrami A, Al-Belushi S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon. 2018;4(10):e00874. doi:10.1016/j.heliyon.2018.e00874
9. Kraut JA, Mullins ME. Toxic Alcohols. N Engl J Med. 2018;378(3):270-80. doi:10.1056/nejmra1615295
10. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J Nutr Biochem. 2002;13(10):572-84. doi:10.1016/s0955-2863(02)00208-5
11. Yuan H, Sun L, Chen M, Wang J. The Simultaneous Analysis of Amadori and Heyns Compounds in Dried Fruits by High Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal Methods. 2017;10:1097-105. doi:10.1007/s12161-016-0669-1
12. Li R, Wei M, Guo G, Li Y, Pan X, Song X, et al. Analysis of Main Components in Jujube and Mulberry Extracts by High-Sensitive HPLC-ESI-Q-TOF-MS/MS. J Chromatogr Sci. 2021;59(9):806-12. doi:10.1093/chromsci/bmaa133
13. Collins PM. Dictionary of Carbohydrates with CD-ROM. 2nd edition. Boca Raton, Florida: Chapman & Hall; 2006.
14. Lang KL, Silva IT, Zimmermann LA, Machado VR, Teixeira MR, Lapuh MI, et al. Synthesis and Cytotoxic Activity Evaluation of Dihydrocucurbitacin B and Cucurbitacin B Derivatives. Bioorg Med Chem. 2012;20(9):3016-30. doi:10.1016/j.bmc.2012.03.001
15. Tannin-Spitz T, Bergman M, Grossman S. Cucurbitacin Glucosides: Antioxidant and Free-Radical Scavenging Activities. Biochem Biophys Res Commun. 2007;364(1):181-6. doi:10.1016/j.bbrc.2007.09.075
16. Shah SSA, Hussain MI, Aslam MK, Rivera G. Natural Products; Pharmacological Importance of Family Cucurbitaceae: A Brief Review. Mini Rev Med Chem. 2014;14(8):694-705. doi:10.2174/1389557514666140820113055
17. Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M. Growth Inhibitory Activity of Cucurbitacin Glucosides Isolated from Citrullus colocynthis on Human Breast Cancer Cells. Biochem Pharmacol. 2007;73(1):56-67. doi:10.1016/j.bcp.2006.09.012
18. Pierson LS, Pierson EA. Roles of Diffusible Signals in Communication among Plant-Associated Bacteria. Phytopathology. 2007;97(2):227-32. doi:10.1094/PHYTO-97-2-0227
19. Ulubelen A, Öztürk M. Alkaloids and Coumarins from Ruta Species. Nat Prod Commun. 2006;1(10):851-7. doi:10.1177/1934578X0600101006
20. Darmadi J, Batubara RR, Himawan S, Azizah NN, Audah HK, Arsianti A, et al. Evaluation of Indonesian mangrove Xylocarpus granatum leaves ethyl acetate extract as potential anticancer drug. Sci Rep. 2021;11:6080. doi:10.1038/s41598-021-85383-3
21. Yang H, Dong Y, Du H, Shi H, Peng Y, Li X. Antioxidant Compounds from Propolis Collected in Anhui, China. Molecules. 2011;16(4):3444-55. doi:10.3390/molecules16043444
Authors
Copyright (c) 2021 Harni Sartika Kamaruddin, Megawati Megawati, Nurliana Nurliana, Carla Wulandari Sabandar

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.