Tentative Identification of Compounds, Antioxidant, and Antimicrobial Activity of the Edible Part of Benincasa hispida L. fruit (Cucurbitaceae)
Abstract
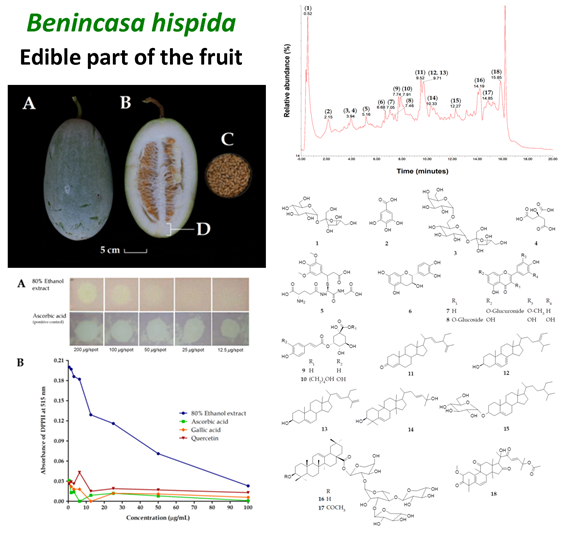
The edible part of Benicasa hispida (Thunb.) Cogn. fruit is traditionally used in Southeast Sulawesi to treat high blood pressure, typhoid fever, and body cooling. The present study evaluated the chemical compounds present in the 80% ethanol of the edible part of the plant using phytochemical screening and an LC-MS analysis, antioxidant activity based on assays on total phenolics content (TPC), total flavonoids content (TFC), and DPPH, and antimicrobial activity towards Salmonella typhi, Escherichia coli, Staphylococcus aureus, and Candida albicans. Phytochemical screening revealed the presence of tannins, flavonoids, terpenoids, steroids, and saponins in the extract. As many as eighteen compounds (1-18) were tentatively identified in the extract, including sugars, a simple phenolic, a tricarboxylic acid, a peptide, flavonoids, quinic acid derivatives, phytosterols, triterpenoids, and saponins. The extract exhibited remarkable antioxidant activity with an SC50 value of 23.4 µg/mL, although its TPC (1.1±0.1 mg GAE/g extract) and TFC (1.0±0.1 mg QE/g extract) values were considered in low amounts. The extract was found inactive to inhibit the microbial growths of all tested microbes. However, raffinose (3) present in the extract might be beneficial as a prebiotic to promote a healthy human gut. The study concludes that the 80% ethanol extract of the edible part of B. hispida fruit could be used to develop natural antioxidant agents and nutraceuticals.
Full text article
References
2. Islam MT, Quispe C, El-Kersh DM, Shill MC, Bhardwaj K, Bhardwaj P, et al. A Literature-Based Update on Benincasa hispida (Thunb.) Cogn.: Traditional Uses, Nutraceutical, and Phytopharmacological Profiles. Oxid Med Cell Longev. 2021;2021:6349041. doi:10.1155/2021/6349041
3. Al-Snafi AE. The Pharmacological Importance of Benincasa hispida. A Review. Int J Pharma Sci Res. 2013;4(12):165-70.
4. Alkawi, Rondonowu SB, Kandou FF. Inventarisasi Tumbuhan Obat dan Pemanfaatannya Secara Tradisional oleh Masyarakat di Desa Amesiu Kabupaten Konawe, Sulawesi Tenggara. Pharmacon. 2021;10(2):790-7. doi:10.35799/pha.10.2021.34026
5. Slamet A, Andarias SH. Studi Etnobotani dan Identifikasi Tumbuhan Berkhasiat Obat Masyarakat Sub Etnis Wolio Kota Baubau Sulawesi Tenggara. Proc Biol Educ Conf. 2018;15(1):721-32.
6. Indrawati, Sabilu Y, Ompo A. Pengetahuan dan Pemanfaatan Tumbuhan Obat Tradisional Masyarakat Suku Moronene di Desa Rau-Rau Sulawesi Tenggara. BioWallacea J Penelitian Biol J Biol Res. 2014;1(1):39-48.
7. Darmayani S, Alaydrus S, Yunus R, Yuniarty T, Dewi NP, Rosanty A, et al. Antibacterial Activities Test of Ethanol Extracts of Kundur Fruit (Benincasa hispida Thunb. Cogn) on Salmonella typhi Bacteria. J Phys Conf Ser. 2021;1899:012028. doi:10.1088/1742-6596/1899/1/012028
8. Sabandar CW, Jalil J, Ahmat N, Aladdin NA, Kamaruddin HS, Wahyuningrum R. Aktivitas Antioksidan dan Penghambatan Xantin Oksidase Kulit Batang Songi (Dillenia serrata Thunb.) J Farmasi Galenika Galenika J Pharm. 2020;6(1):151-9. doi:10.22487/j24428744.2020.v6.i1.15008
9. Kamaruddin HS, Megawati, Nurliana, Sabandar CW. Chemical Constituents and Antioxidant Activity of Melothria scabra Naudin Fruits. Borneo J Pharm. 2021;4(4):283-92. doi:10.33084/bjop.v4i4.2890
10. Sabandar C, Jalil J, Ahmat N, Aladdin NA. Assessment of Antioxidant and Xanthine Oxidase Inhibitory Activity of Triadica cochinchinensis stem bark. Curr Res Biosci Biotech. 2019;1(1):39-44. doi:10.5614/crbb.2019.1.1/HZRA413
11. Sahidin I, Sabandar CW, Wahyuni, Hamsidi R, Mardikasari SA, Zubaydah WOS, et al. Investigation of Compounds and Biological Activity of Selected Indonesian Marine Sponges. Nat Prod J. 2019;10(3):312-21. doi:10.2174/2210315509666190627105237
12. Balouiri M, Sadiki M, Ibnsouda SK. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. J Pharm Anal. 2016;6(2):71-9. doi:10.1016/j.jpha.2015.11.005
13. Zaini NAM, Anwar F, Hamid AA, Saari N. Kundur [Benincasa hispida (Thunb.) Cogn.]: A Potential Source for Valuable Nutrients and Functional Foods. Food Res Int. 2011;44(7):2368-76. doi:10.1016/j.foodres.2010.10.024
14. Jin J, Lao J, Zhou R, He W, Qin Y, Zhong C. Simultaneous Identification and Dynamic Analysis of Saccharides During Stem Processing of Rhizomes of Polygonatum cyrtonema by HPLC-QTOF-MS/MS. Molecules. 2018;23(11):2855. doi:10.3390/molecules23112855
15. Xue S, Wan, X, Lu S, Zhong Y, Xie D. A Time-Course Transciptome Analysis of Wax Gourd Fruit Development Reveals Predominant Genes Regulating Taste and Nutrition. Front Plant Sci. 2022;13:971274. doi:10.3389/fpls.2022.971274
16. Zhang C, Yu X, Ayre BG, Turgeon R. The Origin and Composition of Cucurbit “Phloem” Exudate. Plant Physiol. 2012;158(4);1873-82. doi:10.1104/pp.112.194431
17. Miao M, Zhang Z. Carbohydrate Metabolism of Cucurbits. In: Pessarakli M, editor. Handbook of Cucurbits. Boca Raton, US: CRC Press; 2016. p. 69-91. doi:10.1201/b19233
18. Jiang X, Kuang F, Kong F, Yan C. Prediction of the Antiglycation Activity of Polysaccharides from Benincasa hispida Using a Response Surface Methodology. Carbohydr Polym. 2016;151:358-63. doi:10.1016/j.carbpol.2016.05.079
19. Burel C, Kala A, Purevdorj-Gaje L. Impact of pH on Citric Acid Antimicrobial Activity Against Gram-Negative Bacteria. Lett Appl Microbiol. 2021;72(2):332-40. doi:10.1111/lam.13420
20. Yang H, Wan D, Song F, Liu Z, Liu S. α-Cyano-4-hydroxycinnamic Acid, Sinapinic Acid, and Ferulic Acid as Matrices and Alkylating Agents for Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometric Analysis of Cysteine-Containing Peptides. Rapid Commun Mass Spectrom. 2013;27(12):1410-2. doi:10.1002/rcm.6587
21. Su S, Cui W, Zhou W, Duan J, Shang E, Tang Y. Chemical Fingerprinting and Qualitative Constituent Analysis of Siwu Detection Categorized Formulae by UPLC-QTOF/MS/MS and HPLC-DAD. Chin Med. 2013;8(1):5. doi:10.1186/1749-8546-8-5
22. Mukherjee PK, Singha S, Kar A, Chanda J, Banerjee S, Dasgupta B, et al. Therapeutic Importance of Cucurbitaceae: A Medicinally Important Family. J Ethnopharmacol. 2022;282:14599. doi:10.1016/j.jep.2021.114599
23. Fatariah Z, Zulkhairuazha TYG, Wan Rosli WI. Quantitative HPLC Analysis of Gallic Acid in Benincasa hispida Prepared with Different Extraction Techniques. Sains Malays. 2014;43(8):1181-7.
24. Busuioc AC, Botezatu A-VD, Furdui B, Vinatoru C, Maggi F, Caprioli G, et al. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules. 2020;25(22):5468. doi:10.3390/molecules25225468
25. Du Q, Zhang Q, Ito Y. Isolation and Identification of Phenolic Compounds in the Fruit of Benincasa hispida by HSCCC. J Liq Chromatogr Relat Technol. 2005;28(1):137-44. doi:10.1081/JLC-200038620
26. Xie L, Wang J, Liu F, Zhou H, Chen Y, Pan L, et al. Integrated Analysis of Multiomics and Fine-Mapping Reveals a Candidate Gene Regulating Pericarp Color and Flavonoids Accumulation in Wax Gourd (Benincasa hispida). Front Plant Sci. 2022;13:1019787. doi:10.3389/fpls.2022.1019787
27. Wahid M, Saqib F, Chicea L, Ahmedah HT, Sajer BH, Marc RA. Metabolomics Analysis Delineates the Therapeutic Effects of Hydroethanolic Extract of Cucumis sativus L. Seeds on Hypertension and Isoproterenol-Induced Myocardial Infarction. Biomed Pharmacother. 2022;148:112704. doi:10.1016/j.biopha.2022.112704
28. Rumbero-Sanchez A, Vazquez P. Quinic Acid Esters from Isertia haenkeana. Phytochem. 1991;30(1):311-3. doi:10.1016/0031-9422(91)84144-H
29. Nakano S, Fujimoto Y, Takaishi Y, Osorio C, Duque C. Cucurbita-5,23-diene-3β,25-diol from Sicana odorifera. Fitoterapia. 2004;75(6):609-11. doi:10.1016/j.fitote.2004.05.004
30. Han X, Liu C, Liu Y, Xu Q, Li X, Yang S. New Triterpenoids and Other Constituents from the Fruits of Benincasa hispida (Thunb.) Cogn. J Agric Food Chem. 2013;61(51):12692-9. doi:10.1021/jf405384r
31. Haq FU, Ali A, Khan MN, Shah SMZ, Kandel RC, Aziz N, et al. Metabolite Profiling and Quantitation of Cucurbitacins in Cucurbitaceae Plants by Liquid Chromatography Coupled to Tandem Mass Spectrometry. Sci Rep. 2019;9(1):15992. doi:10.1038/s41598-019-52404-1
32. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid Antioxidants: Chemistry, Metabolism and Structure Activity Relationships. J Nutr Biochem. 2002;13(10):572-84. doi:10.1016/s0955-2863(02)00208-5
33. Hidalgo M, Sánchez-Moreno C, Pascual-Teresa S. Flavonoid-Flavonoid Interaction and Its Effect on Their Antioxidant Activity. Food Chem. 2010;121(3):691-6. doi:10.1016/j.foodchem.2009.12.097
34. Badhani B, Sharma N, Kakkar R. Gallic Acid: A Versatile Antioxidant with Promising Therapeutic and Industrial Applications. RSC Adv. 2015;5:27540-57. doi:10.1039/c5ra01911g
35. Grzesik M, Naparło K, Bartosz G, Sadowska-Bartosz I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018;241:480-92. doi:10.1016/j.foodchem.2017.08.117
36. Hsieh CY, Chag ST. Antioxidant Activities and Xanthine Oxidase Inhibitory Effects of Phenolic Phytochemicals from Acacia confusa Twigs and Branches. J Agric Food Chem. 2010;58(3):1578-83. doi:10.1021/jf903569k
37. Samad NB, Debnath T, Jin HL, Lee BR, Park PJ, Lee SY, et al. Antioxidant Activity of Benincasa hispida Seeds. J Food Biochem. 2013;37(4);388-95. doi:10.1111/j.1745-4514.2011.00643.x
38. Wadikar TS, Setty SB, Bhat KG, Trivedi DJ, Thakur SL. Antibacterial Activity of Aqueous Extract of Benincasa hispida Fruit against Periodontal Pathogens. Int J Sci Study. 2015;3(1):145-9. doi:10.17354/ijss/2015/174
39. Anggraeni AA. Mini-Review: The Potential of Raffinose as a Prebiotic. IOP Conf Ser Earth Environ Sci. 2022;980:012033. doi:10.1088/1755-1315/980/1/012033
40. Daglia M. Polyphenols as Antimicrobial Agents. Curr Opin Biotechnol. 2012;23(2):174-81. doi:10.1016/j.copbio.2011.08.007
Authors
Copyright (c) 2023 Carla Wulandari Sabandar, Harni Sartika Kamaruddin, Reskiya Nur Insani, Rana Triana Amin, Zulkifli Zulkifli, Tien Tien

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.