Clinical Trials: The Role of Regulatory Agencies, Pharmacovigilance Laws, Guidelines, Risk Management, Patenting, and Publicizing Results
Abstract
The research carried out to find a better treatment, improve healthcare, and benefit the current medical practice is termed clinical research. Clinical trial includes the pharmacodynamics (mechanisms of action of a new drug), pharmacokinetics (drug metabolism inside the body), therapeutics (efficacy of the drug), and adverse effects (safety of the drug) of the novel medical products. Clinical research is a process that involves human subjects and their biological specimens. The clinical trial is a meticulously planned protocol-based study of a drug/device to discover a new/better way to prevent, diagnose, and treat a disease/illness. Considering the involvement of both healthy and diseased people in clinical trials, the regulatory authorities have a significant role in the processes involving the conduction of clinical research and carefully evaluate their potential implications on humans. Because clinical trials are usually aimed at assessing the safety and efficacy of novel pharmaceutical compounds and medical devices, pharmacovigilance laws and risk management assume increased significance while conducting clinical research/trials. In this review, we attempt to discuss the regulatory authorities' roles in different geographical regions, including the United States of America, The European Union, and India. We also focus on the importance of pharmacovigilance laws and risk management during clinical trials.
Full text article
References
2. Kandi V, Suvvari TK, Vadakedath S, Godishala V. Microbes, Clinical trials, Drug Discovery, and Vaccine Development: The Current Perspectives. Borneo J Pharm. 2021;4(4):311-23. doi:10.33084/bjop.v4i4.2571
3. Tu YF, Chien CS, Yarmishyn AA, Lin YY, Luo YH, Lin YT, et al. A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int J Mol Sci. 2020;21(7):2657. doi:10.3390/ijms21072657
4. Park JJH, Mogg R, Smith GE, Nakimuli-Mpungu E, Jehan F, Rayner CR, et al. How COVID-19 has fundamentally changed clinical research in global health. Lancet Glob Health. 2021;9(5):e711-20. doi:10.1016/S2214-109X(20)30542-8
5. Bassi A, Arfin S, Joshi R, Bathla N, Hammond NE, Rajbhandari D, et al. Challenges in operationalising clinical trials in India during the COVID-19 pandemic. Lancet Glob Health. 2021;10(3):e317-9. doi:10.1016/s2214-109x(21)00546-5
6. Spigel DR. The value of observational cohort studies for cancer drugs. Biotechnol Healthc. 2010;7(2):18-24.
7. Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1(4):309-26. doi:10.3121/cmr.1.4.309
8. Juneja A, Gupta J, Yadav N, Sharma S, Panchal Y, Adhikari T, et al. An overview of primary registries of WHO's international clinical trial registry platform. Ayu. 2019;40(3):141-6. doi:10.4103/ayu.ayu_62_20
9. Kandi V, Vadakedath S. Clinical Research: An Overview of Study Types, Designs, and Their Implications in the Public Health Perspective. Am J Clin Med Res. 2021;9(2):36-42. doi: 10.12691/ajcmr-9-2-1
10. Lim CY, In J. Randomization in clinical studies. Korean J Anesthesiol. 2019;72(3):221-32. doi:10.4097/kja.19049
11. Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43(2):215-21. doi:10.4085/1062-6050-43.2.215
12. Lin Y, Zhu M, Su Z. The pursuit of balance: An overview of covariate-adaptive randomization techniques in clinical trials. Contemp Clin Trials. 2015;45(Pt A):21-5. doi:10.1016/j.cct.2015.07.011
13. Selukar S, May S, Law D, Othus M. Stratified randomization for platform trials with differing experimental arm eligibility. Clin Trials. 2021;18(5):562-9. doi:10.1177/17407745211028872
14. Yang HL, Wu XB, Mao C. [Block randomization in clinical trials]. Zhonghua Yu Fang Yi Xue Za Zhi. 2019;53(4):437-40. doi:10.3760/cma.j.issn.0253-9624.2019.04.022
15. Park Y. Challenges and opportunities in biomarker-driven trials: adaptive randomization. Ann Transl Med. 2022;10(18):1035. doi:10.21037/atm-21-6027
16. Tavana B, Chen A. Determination of Drugs in Clinical Trials: Current Status and Outlook. Sensors. 2022;22(4):1592. doi:10.3390/s22041592
17. Yan F, Thall PF, Lu KH, Gilbert MR, Yuan Y. Phase I-II clinical trial design: a state-of-the-art paradigm for dose finding. Ann Oncol. 2018;29(3):694-9. doi:10.1093/annonc/mdx795
18. Sramek JJ, Murphy MF, Adcock S, Stark JG, Cutler NR. Phase 1 Clinical Trials of Small Molecules: Evolution and State of the Art. Rev Recent Clin Trials. 2021;16(3):232-41. doi:10.2174/1574887116666210204125844
19. Pascual CD. Clinical Drug Trials: The Path to the Patient. Methods Mol Biol. 2021;2296:411-21. doi:10.1007/978-1-0716-1358-0_24
20. Torres-Saavedra PA, Winter KA. An Overview of Phase 2 Clinical Trial Designs. Int J Radiat Oncol Biol Phys. 2022;112(1):22-9. doi:10.1016/j.ijrobp.2021.07.1700
21. Vadakedath S, Godishala V, Suvvari TK, Kandi V. Pharmacology and Pharmacotherapeutics: Finding New Avenues of Research. Am J Pharmacol Sci. 2021;9(2):46-55. doi:10.12691/ajps-9-2-1
22. Bhatt A. Clinical trials during the COVID-19 pandemic: Challenges of putting scientific and ethical principles into practice. Perspect Clin Res. 2020;11:59-63. doi:10.4103/picr.PICR_77_20
23. Pal M, Berhanu G, Desalegn C, Kandi V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus. 2020;12(3):e7423. doi:10.7759/cureus.7423
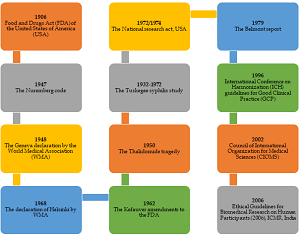
24. Bhatt A. Evolution of clinical research: a history before and beyond james lind. Perspect Clin Res. 2010;1(1):6‐10.
25. Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018;11:156-64. doi:10.1016/j.conctc.2018.08.001
26. Annas GJ. Beyond Nazi War Crimes Experiments: The Voluntary Consent Requirement of the Nuremberg Code at 70. Am J Public Health. 2018;108(1):42-6. doi:10.2105/ajph.2017.304103
27. Muthuswamy V. Ethical issues in clinical research. Perspect Clin Res. 2013;4(1):9-13. doi:10.4103/2229-3485.106369
28. Williamson AE, Burns N. The safety of researchers and participants in primary care qualitative research. Br J Gen Pract. 2014;64(621):198-200. doi:10.3399/bjgp14x679480
29. Collins JF, Sather MR. Regulatory Issues for Clinical Trials in Humans. Epidemiol Rev 2002; 24(1):59-66. doi:10.1093/epirev/24.1.59
30. Bierer BE, White SA, Barnes JM, Gelinas L. Ethical Challenges in Clinical Research During the COVID-19 Pandemic. J Bioeth Inq. 2020;17(4):717-22. doi:10.1007/s11673-020-10045-4
31. Viajayanathan A, Nawawi O. The importance of Good Clinical Practice guidelines and its role in clinical trials. Biomed Imaging Interv J. 2008;4(1):e5. doi:10.2349/biij.4.1.e5
32. Devine S, Dagher RN, Weiss KD, Santana VM. Good clinical practice and the conduct of clinical studies in pediatric oncology. Pediatr Clin North Am. 2008;55(1):187-209. doi:10.1016/j.pcl.2007.10.008
33. Castelino LJ, Narayanan AV, Fernandes SD, Kumar P, Sandeep DS. Good Clinical Practices: An Indian Perspective. Res J Pharm Technol. 2018;11(7):3209-15. doi:10.5958/0974-360X.2018.00590.5
34. Arango J, Chuck T, Ellenberg SS, Foltz B, Gorman C, Hinrichs H, et al. Good Clinical Practice Training: Identifying Key Elements and Strategies for Increasing Training Efficiency. Ther Innov Regul Sci. 2016;50(4):480-6. doi:10.1177/2168479016635220
35. Barkan H. Statistics in clinical research: Important considerations. Ann Card Anaesth. 2015;18(1):74‐82. doi:10.4103/0971-9784.148325
36. Ganasegeran K, Ch'ng ASH, Jamil MFA, Looi I. Clinicians' Perceived Understanding of Biostatistical Results in the Medical Literature: A Cross-Sectional Study. Medicina. 2019;55(6):227. doi:10.3390/medicina55060227
37. Feehan AK, Garcia-Diaz J. Investigator Responsibilities in Clinical Research. Ochsner J. 2020;20(1):44-9. doi:10.31486/toj.19.0085
38. Haller Jr JS. The United States Pharmacopoeia: its origin and revision in the 19th century. Bull N Y Acad Med. 1982;58(5):480-92.
39. National Research Council (US) Committee on the Review of Food and Drug Administration's Role in Ensuring Safe Food. Appendix E: The U.S. Food and Drug Administration and Imported Food Safety. Wallace RB, Oria M, editors. Enhancing Food Safety: The Role of the Food and Drug Administration. Washington: National Academies Press; 2010.
40. Sniffen JC, McFarland LV, Evans CT, Goldstein EJC. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS One. 2018;13(12):e0209205. doi:10.1371/journal.pone.0209205
41. National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Chemical Sciences and Technology; Board on Agriculture and Natural Resources; Board on Life Sciences; Committee on Future Biotechnology Products and Opportunities to Enhance Capabilities of the Biotechnology Regulatory System. The Current Biotechnology Regulatory System. Preparing for Future Products of Biotechnology. Washington: National Academies Press; 2017. doi:10.17226/24605
42. Santoro A, Genov G, Spooner A, Raine J, Arlett P. Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works. Drug Saf. 2017;40(10):855-69. doi:10.1007/s40264-017-0572-8
43. Panteli D, Arickx F, Cleemput I, Dedet G, Eckhardt H, Fogarty E, et al. Pharmaceutical regulation in 15 European countries: Review. Health Syst Transit. 2016;18(5):1–118.
44. Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder DeWan S, et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6(11):e1196-252. doi:10.1016/s2214-109x(18)30386-3
45. Gogtay NJ, Ravi R, Thatte UM. Regulatory requirements for clinical trials in India: What academicians need to know. Indian J Anaesth. 2017;61(3):192-9. doi:10.4103/ija.ija_143_17
46. Singh N, Madkaikar NJ, Gokhale PM, Parmar DV. New drugs and clinical trials rules 2019: Changes in responsibilities of the ethics committee. Perspect Clin Res. 2020;11(1):37-43. doi:10.4103/picr.picr_208_19
47. Chowdhury N, Joshi P, Patnaik A, Saraswathy B. Administrative Structure and Functions of Drug Regulatory Authorities in India. New Delhi: Indian Council for Research on International Economic Relations; 2015.
48. Kalita KN, Kalita A. Psychiatry Update: Psychopharmacology. Guwahati: Department of Psychiatry, Lokopriya Gopinath Bordoloi Regional Institute of Mental Health; 2018.
49. Talbot JC, Nilsson BS. Pharmacovigilance in the pharmaceutical industry. Br J Clin Pharmacol. 1998;45(5):427-31. doi:10.1046/j.1365-2125.1998.00713.x
50. Makwana RG, Desai KV, Kikani V, Vaja MD. Regulatory advances and prospects of variation filing for the registered parenteral products in USA and Europe. Int J Drug Reg Affairs. 2021;9(2):52-5. doi:10.22270/ijdra.v9i2.470
51. Azuma K, Yamanaka S. Recent policies that support clinical application of induced pluripotent stem cell-based regenerative therapies. Regen Ther. 2016;4:36-47. doi:10.1016/j.reth.2016.01.009
52. Singh J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother. 2015;6(3):185-7. doi:10.4103/0976-500x.162004
53. Jadav BH, Thula KC, Maheshwari DG. Regulatory requirements of Pharmacovigilance system and its comparison in India and USA. J Glob Trends Pharm Sci. 2015;6(1):2351-6.
54. Jaani M, Venkatesh ND. Pharmacovigilance-A Master Key for Drug Safety. J Pharm Sci Res. 2019;11(5):1963-70.
55. Saczynski JS, McManus DD, Goldberg RJ. Commonly used data-collection approaches in clinical research. Am J Med. 2013;126(11):946-50. doi:10.1016/j.amjmed.2013.04.016
56. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109-17. doi:10.2165/00002018-199920020-00002
57. Zink RC, Marchenko O, Sanchez-Kam M, Ma H, Jiang Q. Sources of Safety Data and Statistical Strategies for Design and Analysis: Clinical Trials. Ther Innov Regul Sci. 2018;52(2):141-58. doi:10.1177/2168479017738980
58. Große-Michaelis I, Proestel S, Rao RM, Dillman BS, Bader-Weder S, Macdonald L, et al. MedDRA Labeling Groupings to Improve Safety Communication in Product Labels. Ther Innov Regul Sci. 2023;57(1):1-6. doi:10.1007/s43441-022-00393-1
59. Asamah M, Akuffo KO, Nortey P, Donkor N, Danso-Appiah A. Spontaneous reporting of adverse drug reaction among health professionals in Ghana. Arch Public Health. 2022;80(1):33. doi:10.1186/s13690-021-00783-1
60. Salvador MR, Monteiro C, Pereira L, Duarte AP. Quality of Spontaneous Reports of Adverse Drug Reactions Sent to a Regional Pharmacovigilance Unit. Int J Environ Res Public Health. 2022;19(7):3754. doi:10.3390/ijerph19073754
61. Edwards IR. Spontaneous reporting--of what? Clinical concerns about drugs. Br J Clin Pharmacol. 1999;48(2):138-41. doi:Bebayar kah lawn 10.1046/j.1365-2125.1999.00000.x
62. Simundić AM. Bias in research. Biochem Med. 2013;23(1):12-5. doi:10.11613/bm.2013.003
63. Balhara YPS. Indexed journal: What does it mean? Lung India. 2012;29(2):193. doi:10.4103/0970-2113.95345
64. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18(1):5. doi:10.1186/s12874-017-0468-4
65. Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. The Principles of Biomedical Scientific Writing: Abstract and Keywords. Int J Endocrinol Metab. 2020;18(1):e100159. doi:10.5812/ijem.100159
66. Harsoor SS. Art and science of authorship for biomedical research publication. Indian J Anaesth. 2016;60(9):679-83. doi:10.4103/0019-5049.190626
67. Kanankege KST, Phelps NBD,Vesterinen HM, Errecaborde KM, Alvarez J, Bender JB, et al. Lessons Learned From the Stakeholder Engagement in Research: Application of Spatial Analytical Tools in One Health Problems. Front Vet Sci. 2020;7:254. doi:10.3389/fvets.2020.00254
68. Knaggård A, Slunge D, Ekbom A, Göthberg M, Sahlin U. Researchers’ approaches to stakeholders: Interaction or transfer of knowledge? Environ Sci Policy. 2019;97:25-35. doi:10.1016/j.envsci.2019.03.008
69. Maurer M, Mangrum R, Hilliard-Boone T, Amolegbe A, Carman KL, Forsythe L, et al. Understanding the Influence and Impact of Stakeholder Engagement in Patient-centered Outcomes Research: a Qualitative Study. J Gen Intern Med. 2022;37(Suppl 1):6-13. doi:10.1007/s11606-021-07104-w
70. Khadilkar SS. Scientific Misconduct: A Global Concern. J Obstet Gynaecol India. 2018;68(5):331-5. doi:10.1007/s13224-018-1175-8
71. Wager E, Kleinert S. Cooperation between research institutions and journals on research integrity cases: guidance from the committee on publication ethics (cope). Acta Inform Med. 2012;20(3):136-40. doi:10.5455/aim.2012.20.136-140
72. van Raan AF. Patent Citations Analysis and Its Value in Research Evaluation: A Review and a New Approach to Map Technology-relevant Research", J Data Inf Sci. 2017;2(1):13-50. doi:10.1515/jdis-2017-0002
73. Aksnes DW, Langfeldt L, Wouters P. Citations, Citation Indicators, and Research Quality: An Overview of Basic Concepts and Theories. SAGE Open. 2019;9(1):215824401982957. doi:10.1177/2158244019829575
74. Narin, F. Patents as indicators for the evaluation of industrial research output. Scientometrics. 1995;34(3):489-96. doi:10.1007/BF02018015
75. Blind K, Pohlisch J, Aikaterini Z. Publishing, patenting, and standardization: Motives and barriers of scientists. Res Policy. 2018;47(7):1185-97. doi:10.1016/j.respol.2018.03.011
76. Chew BH. Planning and Conducting Clinical Research: The Whole Process. Cureus. 2019;11(2):e4112. doi:10.7759/cureus.4112
77. Banerji A, Suri F. Impact of R and D Intensity, Patents and Regulatory Filings on Export Intensity of Indian Pharmaceutical Industry. Indian J of Pharm Educ Res. 2019;53(4):638-48. doi:10.5530/ijper.53.4.125
78. Ghangas J, Jain N, Sinha A. A critical view of harmonization of regulatory requirements for Generic Drug approval submissions in ASEAN countries. Int J Drug Regul Aff. 2019; 7(1):13-24. doi:10.22270/ijdra. v7i1.297
79. Batta A, Kalra BS, Khirasaria R. Trends in FDA drug approvals over last 2 decades: An observational study. J Family Med Prim Care. 2020;9(1):105‐14. doi:10.4103/jfmpc.jfmpc_578_19
80. Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86(2):140-6. doi:10.2471/blt.07.040089
81. Handoo S, Arora V, Khera D, Nandi PK, Sahu SK. A comprehensive study on regulatory requirements for development and filing of generic drugs globally. Int J Pharm Investig. 2012;2(3):99‐105. doi:10.4103/2230-973X.104392
82. Rana P, Roy V. Generic medicines: issues and relevance for global health. Fundam Clin Pharmacol. 2015;29(6):529‐42. doi:10.1111/fcp.12155
83. Alostad AH, Steinke DT, Schafheutle EI. International Comparison of Five Herbal Medicine Registration Systems to Inform Regulation Development: United Kingdom, Germany, United States of America, United Arab Emirates and Kingdom of Bahrain. Pharmaceut Med. 2018;32(1):39–49. doi:10.1007/s40290-018-0223-0
84. Martin R, Bahmani K. Generic drugs, a need to the public: USA and India – Government plans to reduce the price of abbreviated new drug application and list of generic drugs approved in year 2018. J Generic Med. 2019; 15(3):115-32. doi:10.1177/1741134319848811
85. Bate A, Reynolds RF, Caubel P. The hope, hype, and reality of Big Data for pharmacovigilance. Ther Adv Drug Saf. 2018;9(1):5‐11. doi:10.1177/2042098617736422
Authors
Copyright (c) 2023 Venkataramana Kandi, Sabitha Vadakedath, Purna Singh Addanki, Vikram Godishala, Venkata Bharatkumar Pinnelli

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.