In-Silico Design and Evaluation of the Anti-Wolbachia Potential of Boron-Pleuromutilins
Abstract
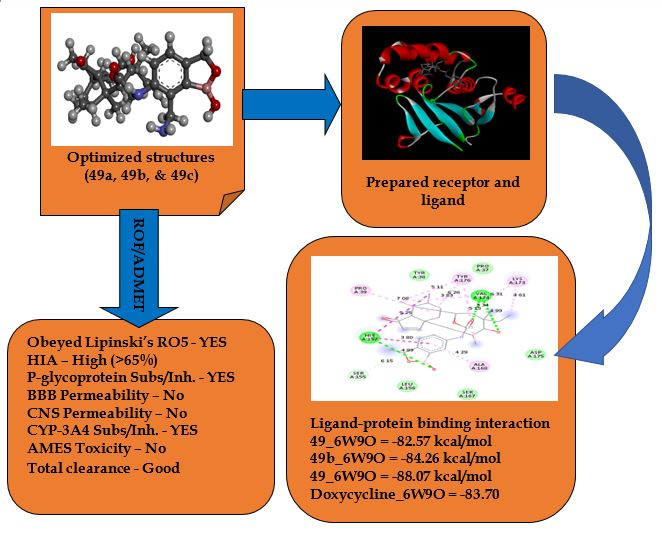
Filariasis (Lymphatic filariasis and Onchocerciasis) is a common neglected tropical disease caused by parasitic nematodes called filarial worms, which often host the Wolbachia bacteria. A good treatment approach seeks Wolbachia as a drug target. Here, a computer-aided design of some boron-pleuromutilin analogs was conducted using the ligand-based drug design approach while performing molecular docking investigation and pharmacokinetics analyses to evaluate their drug-likeness properties. The newly designed compounds (49a, 49b, and 49c) showed improved inhibitory activities (pEC50) over those of the template and the clinically relevant pleuromutilins (retapamulin and lefamulin) in the order; 49b (pEC50 = 9.0409) > 49c (8.8175) > 49a (8.5930) > template (49) (8.4222) > retapamulin (6.7403) > lefamulin (6.1369). Standard docking performed with OTU deubiquitinase (6W9O) revealed the order of binding energies; 49c (-88.07 kcal/mol) > 49b (-84.26 kcal/mol) > doxycycline (-83.70 kcal/mol) > template (-82.57 kcal/mol) > 49a (-78.43 kcal/mol) > lefamulin (-76.83 kcal/mol) > retapamulin (-76.78 kcal/mol), with the new compounds all showing good pharmacological interactions with the receptor’s amino acids. The new analogs were also predicted to be orally bioavailable with better pharmacokinetic profiles than the template, retapamulin, lefamulin, and doxycycline having no more than one violation of Lipinski’s ROF. Therefore, the newly designed compounds could be considered potential anti-filarial drug candidates.
Full text article
References
2. Bakowski MA, Shiroodi RK, Liu R, Olejniczak J, Yang B, Gagaring K, et al. Discovery of short-course antiwolbachial quinazolines for elimination of filarial worm infections. Sci Transl Med. 2019;11(491):eaav3523. doi: 10.1126/scitranslmed.aav3523
3. Jacobs RT, Lunde CS, Freund YR, Hernandez V, Li X, Xia Y, et al. Boron-pleuromutilins as anti-Wolbachia agents with potential for treatment of onchocerciasis and lymphatic filariasis. J Med Chem. 2019;62(5):2521-40. doi:10.1021/acs.jmedchem.8b01854
4. Carter DS, Jacobs RT, Freund YR, Berry PW, Akama T, Easom EE, et al. Macrofilaricidal Benzimidazole–Benzoxaborole Hybrids as an Approach to the Treatment of River Blindness: Part 2. Ketone Linked Analogs. ACS Infect Dis. 2019;26;6(2):180-5. doi:10.1021/acsinfecdis.9b00397
5. Lakshmi V, Joseph SK, Srivastava S, Verma SK, Sahoo MK, Dube V, et al. Antifilarial activity in vitro and in vivo of some flavonoids tested against Brugia malayi. Acta Trop. 2010;116(2):127-33. doi:10.1016/j.actatropica.2010.06.006
6. Sashidhara KV, Rao KB, Kushwaha V, Modukuri RK, Verma R, Murthy PK. Synthesis and antifilarial activity of chalcone–thiazole derivatives against a human lymphatic filarial parasite, Brugia malayi. Eur J Med Chem. 2014;81:473-80. doi:10.1016/j.ejmech.2014.05.029
7. Slatko BE, Taylor MJ, Foster JM. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis. 2010;51(1):55-65. doi:10.1007/s13199-010-0067-1
8. Bouchery T, Lefoulon E, Karadjian G, Nieguitsila A, Martin C. The symbiotic role of Wolbachia in Onchocercidae and its impact on filariasis. Clin Microbiol Infect. 2013;19(2):131-40. doi:10.1111/1469-0691.12069
9. Bakowski MA, McNamara CW. Advances in Antiwolbachial Drug Discovery for Treatment of Parasitic Filarial Worm Infections. Trop Med Infect Dis. 2019;4(3):108. doi:10.3390/tropicalmed4030108
10. Bailey-Elkin BA, van Kasteren PB, Snijder EJ, Kikkert M, Mark BL. Viral OTU deubiquitinases: a structural and functional comparison. PLoS Pathog. 2014;10(3):e1003894. doi:10.1371/journal.ppat.1003894
11. Schubert AF, Nguyen JV, Franklin TG, Geurink PP, Roberts CG, Sanderson DJ, et al. Identification and characterization of diverse OTU deubiquitinases in bacteria. EMBO J. 2020;39(15):e105127. doi:10.15252/embj.2020105127
12. Ugbe FA, Shallangwa GA, Uzairu A, Abdulkadir I. Molecular Docking Screening and Pharmacokinetic Studies of Some Boron-Pleuromutilin Analogues against Possible Targets of Wolbachia pipientis. J Mol Docking. 2022;2(1):29-43. doi:10.33084/jmd.v2i1.3450
13. Newman DJ, Cragg GM. Natural products of therapeutic importance. Compr Nat Prod II. 2010;2:623–50. doi:10.1016/b978-008045382-8.00055-1
14. Dasenaki ME, Kritikou AS, Thomaidis NS. Chapter 17 - Meat safety: II Residues and contaminants. In Lawrie's Meat Science (Ninth Edition). Cambridge: Woodhead Publishing; 2023. pp. 591-626. doi:10.1016/B978-0-323-85408-5.00007-8
15. Brown P, Dawson MJ. A perspective on the next generation of antibacterial agents derived by manipulation of natural products. Prog Med Chem. 2015;54:135-84. doi:10.1016/bs.pmch.2014.10.001
16. Ugbe FA, Shallangwa GA, Uzairu A, Abdulkadir I. Activity modeling, molecular docking and pharmacokinetic studies of some boron-pleuromutilins as anti-wolbachia agents with potential for treatment of filarial diseases. Chem Data Collect. 2021;36:100783. doi:10.1016/j.cdc.2021.100783
17. Adeniji SE, Arthur DE, Abdullahi M, Abdullahi A, Ugbe FA. Computer-aided modeling of triazole analogues, docking studies of the compounds on DNA gyrase enzyme and design of new hypothetical compounds with efficient activities. J Biomol Struct Dyn. 2022;40(9):4004-20. doi:10.1080/07391102.2020.1852963
18. Ugbe FA, Shallangwa GA, Uzairu A, Abdulkadir I. Theoretical modeling and design of some pyrazolopyrimidine derivatives as Wolbachia inhibitors, targeting lymphatic filariasis and onchocerciasis. In Silico Pharmacol. 2022;10(1):8. doi:10.1007/s40203-022-00123-3
19. Pires DE, Blundell TL, Ascher DB. pkCSM: predicting small-molecule pharmacokinetic properties using graph-based signatures. J Med Chem. 2015;58(9):4066-72. doi:10.1021/acs.jmedchem.5b00104
20. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi:10.1038/srep42717
21. Edache EI, Uzairu A, Mamza PA, Shallangwa GA. QSAR, homology modeling, and docking simulation on SARS-CoV-2 and pseudomonas aeruginosa inhibitors, ADMET, and molecular dynamic simulations to find a possible oral lead candidate. J Genet Eng Biotechnol. 2022;20:88. doi:10.1186/s43141-022-00362-z
22. Wang X, Dong H, Qin Q. QSAR models on aminopyrazole-substituted resorcylate compounds as Hsp90 inhibitors. J Comput Sci Eng. 2020;48:1146-56.
23. Li Z, Wan H, Shi Y, Ouyang P. Personal experience with four kinds of chemical structure drawing software: review on ChemDraw, ChemWindow, ISIS/Draw, and ChemSketch. J Chem Inf Comput Sci. 2004;44(5):1886-90. doi:10.1021/ci049794h
24. Ugbe FA, Shallangwa GA, Uzairu A, Abdulkadir I. Theoretical activity prediction, structure-based design, molecular docking and pharmacokinetic studies of some maleimides against Leishmania donovani for the treatment of leishmaniasis. Bull Natl Res Cent. 2022;46:92. doi:10.1186/s42269-022-00779-z
25. Edache EI, Uzairu A, Mamza PA, Shallangwa GA. Theoretical Investigation of the Cooperation of Iminoguanidine with the Enzymes-Binding Domain of Covid-19 and Bacterial Lysozyme Inhibitors and their Pharmacokinetic Properties: Iminoguanidine Derivatives as Multi-target Lead Compound Against Covid-19 and Pseudomonas aeruginosa. J Mex Chem Soc. 2022;66(4):513-42. doi:10.29356/jmcs.v66i4.1726
26. Arun K, Sharmila R, Akila K, Jaikumar B. In-silico approach for the assessment of oral cancer property on Limonia acidissima. Int J Pharm Sci Res. 2016;7(3):1271-5. doi:10.13040/ijpsr.0975-8232.7(3).1271-75
27. Amin SA, Gayen S. Modelling the cytotoxic activity of pyrazolo-triazole hybrids using descriptors calculated from the open source tool “PaDEL-descriptor”. J Taibah Univ Sci. 2016;10(6):896-905. doi:10.1016/j.jtusci.2016.04.009
28. Aniyery RB, Gupta A, Singh P. In-vitro and in silico antimicrobial study of stannane of pyridoxal 5-phosphate. Int J Pharm Pharm Sci. 2017;9(2):145-53. doi:10.22159/ijpps.2017v9i2.15002
29. Nipun TS, Khatib A, Ibrahim Z, Ahmed QU, Redzwan IE, Primaharinastiti R, et al. GC-MS-and NMR-based metabolomics and molecular docking reveal the potential alpha-glucosidase inhibitors from psychotria malayana jack leaves. Pharmaceuticals. 2021;14(10):978. doi:10.3390/ph14100978
30. Imberty A, Hardman KD, Carver JP, Perez S. Molecular modelling of protein-carbohydrate interactions. Docking of monosaccharides in the binding site of concanavalin A. Glycobiology. 1991;1(6):631-42. doi:10.1093/glycob/1.6.631
31. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1-3):3-26. doi:10.1016/s0169-409x(00)00129-0
32. Pratama MRF, Poerwono H, Siswodiharjo S. ADMET properties of novel 5-O-benzoylpinostrobin derivatives. J Basic Clin Physiol Pharmacol. 2019;30(6):20190251. doi:10.1515/jbcpp-2019-0251
33. Abdul-Hammed M, Adedotun IO, Olajide M, Irabor CO, Afolabi TI, Gbadebo IO, et al. Virtual screening, ADMET profiling, PASS prediction, and bioactivity studies of potential inhibitory roles of alkaloids, phytosterols, and flavonoids against COVID-19 main protease (Mpro). Nat Prod Res. 2022;36(12):3110-6. doi:10.1080/14786419.2021.1935933
Authors
Copyright (c) 2023 Fabian Audu Ugbe, Gideon Adamu Shallangwa, Adamu Uzairu, Ibrahim Abdulkadir

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.