2D-QSAR, Docking, Molecular Dynamics Simulations with the MM/GBSA Approaches against Graves' Disease and PTPN22
Abstract
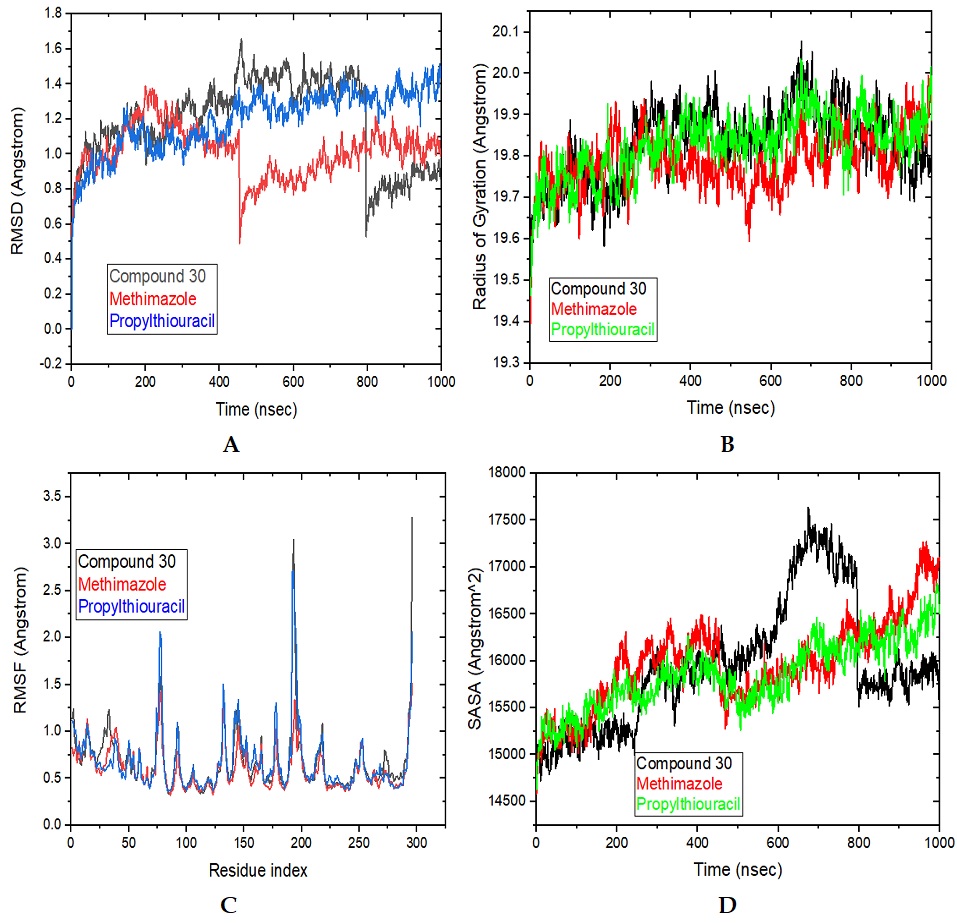
Graves' disease (GD) is an autoimmune condition that frequently causes hyperthyroidism and thyrotoxicosis. Protein tyrosine phosphatase, non-receptor type 22 (lymphoid) isoform 1 (PTPN22), is a promising therapeutic candidate for treating GD, rheumatoid arthritis, type 1 diabetes, and other autoimmune disorders. In this dataset, 31 molecular compounds and two standard drugs were optimized using the semi-empirical PM7 theory method via MOPAC v22.0.4 to reveal the key influencing factors contributing to their grave's disease inhibition activity and selectivity. Using QSARIN software, the acquired properties/descriptors were used to create a quantitative structural activities relationship (QSAR) model, and the similarities between the observed and predicted pIC50 values were examined. A molecular docking simulation study also uncovers non-covalent interactions between the investigated compounds and the receptors. The observed ligand-protein interactions with GD proteins (PDB ID 2XPG and 4QT5) and PTPN22 (PDB ID 3BRH) were investigated. The pharmacokinetics (ADMET) properties were also investigated. Finally, molecular dynamics (MD) simulation and MM/GBSA studies that demonstrated stable trajectory and molecular properties with a consistent interaction profile were used to validate the stability of the compounds in the complex with PTPN22.
Full text article
References
2. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet. 2016;388(10047):906-18. doi:10.1016/s0140-6736(16)00278-6
3. Delhasse S, Debove I, Arnold-Kunz G, Ghika JA, Chabwine JN. Erratic movement disorders disclosing Graves' disease and paralleling thyroid function but not autoantibody levels. J Int Med Res. 2019;47(3):1378-86. doi:10.1177/0300060518816873
4. Subekti I, Pramono LA. Current Diagnosis and Management of Graves’ Disease. Acta Med Indones Indones J Intern Med. 2018;50(2):177-82.
5. Ginsberg J. Diagnosis and management of Graves' disease. CMAJ. 2003;168(5):575-85.
6. Li Z, Cestari DM, Fortin E. Thyroid eye disease: what is new to know? Curr Opin Ophthalmol. 2018;29(6):528-34. doi:10.1097/icu.0000000000000529
7. Wémeau JL, Klein M, Sadoul JL, Briet C, Vélayoudom-Céphise FL. Graves' disease: Introduction, epidemiology, endogenous and environmental pathogenic factors. Ann Endocrinol. 2018;79(6):599-607. doi:10.1016/j.ando.2018.09.002
8. Kunc M, Gabrych A, Witkowski JM. Microbiome impact on metabolism and function of sex, thyroid, growth and parathyroid hormones. Acta Biochim Pol. 2016;63(2):189–201. doi:10.18388/abp.2015_1093
9. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–95. doi:10.1111/joim.12395
10. Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, et al. Graves’ disease. Nat Rev Dis Primers. 2020;6(1):52. doi:10.1038/s41572-020-0184-y
11. Edache EI, Samuel H, Sulyman YI, Arinze O, Ayine OI. QSAR and Molecular Docking Analysis of Substituted Tetraketone and Benzyl-benzoate Analogs as Anti-tyrosine: A Novel Approach to anti-tyrosine kinase Drug Design and Discovery. Chem Res J. 2020;5(6):79-100.
12. Ugbe FA, Shallangwa GA, Uzairu A, Abdulkadir I. A combined 2‑D and 3‑D QSAR modeling, molecular docking study, design, and pharmacokinetic profiling of some arylimidamide‑azole hybrids as superior L. donovani inhibitors. Bull Natl Res Cent. 2022;46:189. doi:10.1186/s42269-022-00874-1
13. Abdullahi M, Uzairu A, Shallangwa GA, Mamza PA, Ibrahim MT. 2D-QSAR, 3D-QSAR, molecular docking and ADMET prediction studies of some novel 2-((1H-indol-3-yl)thio)-N-phenyl-acetamide derivatives as anti-influenza A virus. Egypt J Basic Appl Sci. 2022;9(1):510-32. doi:10.1080/2314808X.2022.2108592
14. Edache EI, Uzairu A, Mamza PA, Shallangwa GA. Structure-based simulated scanning of rheumatoid arthritis inhibitors: 2D-QSAR, 3D-QSAR, docking, molecular dynamics simulation, and lipophilicity indices calculation. Sci Afr. 2022;15:e01088. doi:10.1016/j.sciaf.2021.e01088
15. Stewart JJP. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-optimization of Parameters", J Mol Mod. 2013;19:1-32. doi:10.1007/s00894-012-1667-x
16. Yap CW. PaDEL-Descriptor: An open source software to calculate molecular descriptors and fingerprints, J Comput Chem. 2011;32(7):1466–74. doi:10.1002/jcc.21707
17. Gramatica P, Chirico N, Papa E, Cassani S, Kovarich S. QSARINS: A New Software for the Development, Analysis, and Validation of QSAR MLR Models, J Comput Chem. 2013;34(24):2121–32. doi:10.1002/jcc.23361
18. Edache EI, Hambali HU, Arthur DE, Oluwaseye A, Chinweuba OC. In-silico Discovery and Simulated Selection of Multi-target Anti-HIV-1 Inhibitors. Int Res J Pure Appl Chem. 2016;11(1):1-15. doi:10.9734/IRJPAC/2016/22863
19. Valdes-Tresanco MS, Valdes-Tresanco ME, Valiente PA, Moreno E. AMDock: a versatile graphical tool for assisting molecular docking with Autodock Vina and Autodock4. Biol Direct. 2020;15(1):12. doi:10.1186/s13062-020-00267-2
20. Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J Comput Chem. 2010;31(2):455-61. doi:10.1002/jcc.21334
21. Harris R, Olson AJ, Goodsell DS. Automated prediction of ligand-binding sites in proteins, Proteins. 2007;70(4);1506–17. doi:10.1002/prot.21645
22. Feinstein WP, Brylinski M. Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets, J Cheminform. 2015;7:18. doi:10.1186/s13321-015-0067-5
23. McMahon RM, Friis L, Siebold C, Friese MA, Fugger L, Jones EY. Structure of HLA-A*0301 in complex with a peptide of proteolipid protein: insights into the role of HLA-A alleles in susceptibility to multiple sclerosis. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 5):447-54. doi:10.1107/s0907444911007888
24. Chen CR, Hubbard PA, Salazar LM, Mclachlan SM, Murali R, Rapoport B. Crystal structure of a TSH receptor monoclonal antibody: insight into graves' disease pathogenesis. Mol Endocrinol. 2015;29(1):99-107. doi:10.1210/me.2014-1257
25. Seidel R, Love J, Piserchio A, Cowburn D. Protein Tyrosine Phosphatase PTPN-22 (Lyp) bound to the mono-Phosphorylated Lck active site peptide. New Jersey (US): RCSB Protein Data Bank; 2009. Available from: https://www.rcsb.org/structure/3BRH
26. Pratama MRF, Poerwono H, Siswodiharjo S. ADMET properties of novel 5-O-benzoylpinostrobin derivatives. J Basic Clin Physiol Pharmacol. 2019;30(6):20190251. doi:10.1515/jbcpp-2019-0251
27. Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Sci Rep. 2017;7:42717. doi:10.1038/srep42717
28. Lee J, Cheng X, Swails JM, Yeom MS, Eastman PK, Lemkul, JA, et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J Chem Theory Comput. 2016;12(1):405–13. doi:10.1021/ACS.JCTC.5B00935
29. Huang J, Rauscher S, Nawrocki G, Ran T, Feig M, De Groot BL, et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat Methods. 2016;14(1):71–3. doi:10.1038/nmeth.4067
30. Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, et al. Scalable Molecular Dynamics with NAMD. J Comput Chem. 2005;26(16):1781–802. doi:10.1002/jcc.20289
31. Humphrey W, Dalke A, Schulten K. VMD-visual molecular dynamics. J Mol Graph. 1996;14(1):33–8. doi:10.1016/0263-7855(96)00018-5
32. Bai Q, Tan S, Xu T, Liu H, Huang J, Yao X. MolAICal: a soft tool for 3D drug design of protein targets by artificial intelligence and classical algorithm. Brief Bioinform. 2021;22(3):bbaa161. doi:10.1093/bib/bbaa161
33. Edache EI, Uzairu A, Mamza PA, Shallangwa GA. Docking Simulations and Virtual Screening to find Novel Ligands for T3S in Yersinia pseudotuberculosis YPIII, A drug target for type III secretion (T3S) in the Gram-negative pathogen Yersinia pseudotuberculosis. Chem Rev Lett. 2021;4(3):130-44. doi:10.22034/crl.2021.254804.1088
34. Erdogan T. DFT, molecular docking and molecular dynamics simulation studies on some newly introduced natural products for their potential use against SARS-CoV-2, J Mol Struct. 2021;1242:130733. doi:10.1016/j.molstruc.2021.130733
35. Lipinski CA. Lead, and drug-like compounds: the rule-of-five revolution. Drug Discov Today, 2004;1(4):337-41. doi:10.1016/j.ddtec.2004.11.007
36. Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J Med Chem. 2002;45(12):2615–23. doi:10.1021/jm020017n
Authors
Copyright (c) 2023 Emmanuel Israel Edache, Adamu Uzairu, Paul Andrew Mamza, Gideon Adamu Shallangwa

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.