Chemical Compound Profile of Bajakah Kalalawit (Uncaria gambir Roxb) Stem Extract Using Liquid Chromatography High-Resolution Mass Spectrometry
Abstract
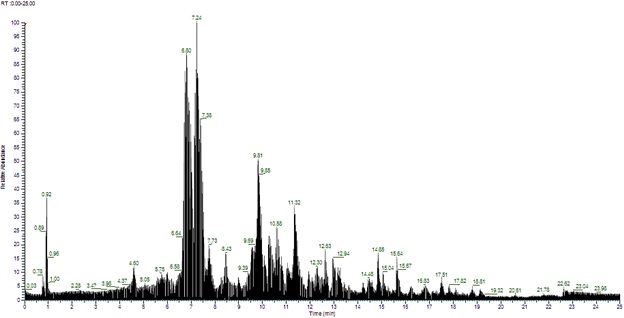
Uncaria gambir Roxb., commonly known as Bajakah Kalalawit, is a plant endemic to Kalimantan, Indonesia, with potential medicinal properties. While previous studies have investigated the phytochemical composition of U. gambir leaves, limited information exists regarding the constituents of its stem extract. This study aimed to comprehensively characterize the chemical composition of U. gambir stem extract using Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS). Ethanol (96%) was employed as the solvent for maceration extraction (1 : 5, plant material : solvent ratio) for three days. Subsequently, the dried extract was subjected to LC-HRMS analysis. Compounds with an Area Under the Curve (AUC) value greater than or equal to 1% were considered as major constituents. The results revealed the presence of 18 distinct chemical compounds in the U. gambir stem extract, providing valuable insights into its phytochemical profile and laying the foundation for further investigations into its potential pharmacological activities.
Full text article
References
2. Vaou N, Stavropoulou E, Voidarou CC, Tsakris Z, Rozos G, Tsigalou C, et al. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics. 2022;11(8):1014. DOI: 10.3390/antibiotics11081014; PMCID: PMC9404952; PMID: 36009883
3. Nurkhasanah, Minangsari DNI, Yulianny VA. The Combination of Rosella (Hibiscus sabdariffa, L) and Stevia (Stevia rebaudiana) Extracts Increase the Antioxidant Activity and Stability. Int J Pharm Pharm Sci. 2016;8(5):16–7.
4. Giordano D, Biancaniello C, Argenio MA, Facchiano A. Drug Design by Pharmacophore and Virtual Screening Approach. Pharmaceuticals. 2022;15(5):646. DOI: 10.3390/ph15050646; PMCID: PMC9145410; PMID: 35631472
5. Rollando R, Ardanareswari A, Susanto FH, Monica E. Efek Afrodisiaka dari Ekstrak Batang Bajakah Kalalawit (Uncaria gambir Roxb.) terhadap Tikus Jantan Galur Wistar (Rattus novergicus). J Pharmascience. 2022;9(2):213-24. DOI: 10.20527/jps.v9i2.13289
6. Nurmiati, Rollando, Susanto FXH. Uji Toksisitas Ekstrak Batang Tumbuhan Bajakah Kalalawit (Uncaria Gambir Roxb.) Pada Organ Ginjal Hewan Tikus Putih Jantan Galur Wistar. Sainsbertek J Ilmu Sains Teknol. 2020;1(1):277-304.
7. Munggari IP, Kurnia D, Deawati Y, Julaeha E. Current Research of Phytochemical, Medicinal and Non-Medicinal Uses of Uncaria gambir Roxb.: A Review. Molecules. 2022;27(19):6551. DOI: 10.3390/molecules27196551; PMCID: PMC9571117; PMID: 36235088
8. Frethernety A, Jelita H, Nugrahini S. Potensi Bahan Alam di Kalimantan Tengah Sebagai Antikariogenik. Yogyakarta: Jejak Pustaka; 2023.
9. Wathan N, Safarina NR, Fitriana M. Aktivitas antioksidan dan kandungan flavonoid total ekstrak etanol batang bajakah kalalawit (Uncaria cordata (Lour) Merr.) asal Kecamatan Loksado Kalimantan Selatan. Sasambo J Pharm. 2024;5(1):1–8. DOI: 10.29303/sjp.v5i1.230
10. Mushtaq Z, Hussain M, Saeed F, Imran A, Umar M, Abdelgawad MA, et al. Asiatic Acid: A Review on Its Polypharmacological Properties and Therapeutic Potential Against Various Maladies. Int J Food Prop. 2023;26(1):1244–63. DOI: 10.1080/10942912.2023.2209702
11. Gray NE, Chamberlin SR, Brandes MS, Brumbach BH, Quinn JF. Asiatic Improves Brain Mitochondrial Function, Activates Antioxidant Response and Attenuates Cognitive Deficits in Beta Amyloid Overexpressing Mice. Alzheimers Dement. 2023;19(57):e062432. DOI: 10.1002/alz.062432
12. Li L, Wang WJ, Xu Q, Huang M. Asiatic Acid Improves Insulin Secretion Of Β Cells in Type 2 Diabetes Through Tnf-Α/Mfn2 Pathway. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023;52(2):185–94. DOI: 10.3724/zdxbyxb-2022-0647; PMCID: PMC10409975; PMID: 37283103
13. Fong LSF, Razali NNM, Ng CT, Yong YK, Hakim MN, Lim YM. Asiatic Acid Inhibits Pro-Oxidant Mediators-Induced Oxidative Stress in Human Aortic Endothelial Cells. Planta Med. 2022;88(15):1471. DOI: https://dx.doi.org/10.1055/s-0042-1759091
14. Lai YW, Wang SW, Lin CL, Chen SS, Lin KH, Lee YT, et al. Asiatic Acid Exhibits Antimetastatic Activity in Human Prostate Cancer Cells by Modulating the Mzf-1/Elk-1/Snail Signaling Axis. Eur J Pharmacol. 2023;951:175770. DOI: 10.1016/j.ejphar.2023.175770; PMID: 37209940
15. Sharma R, Guru SK, Jain SK, Pathania AS, Vishwakarma RA, Bhushan S, et al. 3-(2,6-Dichloro-Benzyloxy)-11-Oxo-Olean-12-Ene-29-Oic Acid, A Semisynthetic Derivative of Glycyrrhetic Acid: Synthesis, Antiproliferative, Apoptotic and Anti-Angiogenesis Activity. Med Chem Commun. 2015;6(4):564–75. DOI: 10.1039/C4MD00344F
16. Herawati E, Novalia K. Gambaran Pengetahuan Lansia di Desa Banaran, Kabupaten Nganjuk tentang Manfaat Seledri bagi Kesehatan Sistem Urinaria. J Nusantara Med. 2022;5(2):31–6. DOI: 10.29407/judika.v5i2.17406
17. Chen J, Xu YH, Yang Y, Yao X, Fu YH, Wang Y, et al. Evaluation of the Anticancer Activity and Mechanism Studies of Glycyrrhetic Acid Derivatives toward HeLa Cells. Molecules. 2023;28(7):3164. DOI: 10.3390/molecules28073164; PMCID: PMC10095686; PMID: 37049928
18. Zhao B, Tomoda Y, Mizukami H, Makino T. 9-Oxo-(10E,12E)-octadecadienoic acid, a cytotoxic fatty acid ketodiene isolated from eggplant calyx, induces apoptosis in human ovarian cancer (HRA) cells. J Nat Med. 2015;69(3):296–302. DOI: 10.1007/s11418-015-0892-x; PMID: 25724148
19. Ko YC, Choi HS, Kim SL, Yun BS, Lee DS. Anti-Inflammatory Effects of (9Z,11E)-13-Oxooctadeca-9,11-dienoic Acid (13-KODE) Derived from Salicornia herbacea L. on Lipopolysaccharide-Stimulated Murine Macrophage via NF-kB and MAPK Inhibition and Nrf2/HO-1 Signaling Activation. Antioxidants. 2022;11(2):180. DOI: 10.3390/antiox11020180; PMCID: PMC8868157; PMID: 35204063
20. Wisitpongpun P, Potup P, Usuwanthim K. Oleamide-Mediated Polarization of M1 Macrophages and IL-1β Production by Regulating NLRP3-Inflammasome Activation in Primary Human Monocyte-Derived Macrophages. Front Immunol. 2022;13:856296. DOI: 10.3389/fimmu.2022.856296; PMCID: PMC9062104; PMID: 35514993
21. Yerlikaya S. Oleamide, A Sleep-Inducing Compound: Effects on Ion Channels and Cancer. Bioelectricity. 2022;4(3):136–44. DOI: 10.1089/bioe.2022.0010
22. Kobayashi Y, Watanabe N, Kitakaze T, Sugimoto K, Izawa T, Kai K, et al. Oleamide Rescues Tibialis Anterior Muscle Atrophy of Mice Housed in Small Cages. Br J Nutr. 2021;126(4):481–91. DOI: 10.1017/s0007114520004304; PMID: 33143796
23. Nunes EA, Rafacho A. Implications of Palmitoleic Acid (Palmitoleate) On Glucose Homeostasis, Insulin Resistance and Diabetes. Curr Drug Targets. 2017;18(6):619–28. DOI: 10.2174/1389450117666151209120345; PMID: 26648072
24. Tovar R, Gavito AL, Vargas A, Soverchia L, Hernandez-Folgado L, Jagerovic N, et al. Palmitoleoylethanolamide Is an Efficient Anti-Obesity Endogenous Compound: Comparison with Oleylethanolamide in Diet-Induced Obesity. Nutrients. 2021;13(8):2589. DOI: 10.3390/nu13082589; PMCID: PMC8400335; PMID: 34444748
25. Muthuraj PG, Pattnaik A, Sahoo PK, Islam T, Pattnaik AK, Byrareddy SN, et al. Palmitoleate Protects Against Zika Virus-Induced Placental Trophoblast Apoptosis. Biomedicines. 2021;9(6):643. DOI: 10.3390/biomedicines9060643; PMCID: PMC8226770; PMID: 34200091
26. Souza CO, Teixeira AADS, Lima EA, Batatinha H, Hirabara SM, Festuccia WT, et al. Palmitoleate Attenuates Diet Induced Insulin Resistance and Hepatic Inflammation Independently of PPAR-α. Cancer Metab. 2014;2(Suppl 1):P52. DOI: 10.1186/2049-3002-2-S1-P52; PMCID: PMC4073097
27. Ragucci S, Bulgari D, Landi N, Russo R, Clemente A, Valletta M, et al. The Structural Characterization and Antipathogenic Activities of Quinoin, A Type 1 Ribosome-Inactivating Protein from Quinoa Seeds. Int J Mol Sci. 2021;22(16):8964. DOI: 10.3390/ijms22168964; PMCID: PMC8396469; PMID: 34445686
28. Pineda B, de la Cruz VP La, Pando RH, Sotelo J. Quinacrine As A Potential Treatment for Covid-19 Virus Infection. Eur Rev Med Pharmacol. 2020;25(1):556–66. DOI: 10.26355/eurrev_202101_24428; PMID: 33506949
29. Bian J, Kang HE, Telling GC. Quinacrine Promotes Replication and Conformational Mutation of Chronic Wasting Disease Prions. Proc Natl Acad Sci U S A. 2014; 111(16):6028-33. DOI: 10.1073/pnas.1322377111; PMCID: PMC4000840; PMID: 24711410
30. Rajendram R, Rajendram R, Preedy VR. Eating, Drinking, Smoking and Cancer Prevention: A Focus on Acetaldehyde. In: Fang EF, Ng TB, editors. Antitumor Potential and other Emerging Medicinal Properties of Natural Compounds. Dordrecht: Springer; 2013. p. 294–62. DOI: 10.1007/978-94-007-6214-5_17
31. Virdis P, Marchesi I, Fiorentino F, Migheli R, Sanna L, Bordoni V, et al. Tomentosin a Sesquiterpene Lactone Induces Antiproliferative and Proapoptotic Effects in Human Burkitt Lymphoma by Deregulation of Anti- and Pro-Apoptotic Genes. Life. 2021;11(11):1128. DOI: 10.3390/life11111128; PMCID: PMC8623649; PMID: 34833004
32. Yang H, Zhao H, Dong X, Yang Z, Chang W. Tomentosin induces apoptotic pathway by blocking inflammatory mediators via modulation of cell proteins in AGS gastric cancer cell line. J Biochem Mol Toxixology. 2020;34(8):e22501. DOI: 10.1002/jbt.22501; PMID: 32227673
33. Fan Y, Maghimaa M, Chinnathambi A, Alharbi SA, Veeraraghavan VP, Mohan SK, et al. Tomentosin Reduces Behavior Deficits and Neuroinflammatory Response in MPTP-Induced Parkinson's Disease in Mice. J Environ Pathol Toxicol. 2019;40(1):75–84. DOI: 10.1615/jenvironpatholtoxicoloncol.v40.i1.70; PMID: 33639075
34. Yang S, Park SH, Oh SW, Kwon K, Yu E, Lee CW, et al. Antioxidant Activities and Mechanisms of Tomentosin In Human Keratinocytes. Antioxidants J. 2022;11(5):990. DOI: 10.3390/antiox11050990; PMCID: PMC9137523; PMID: 35624854
35. Park HH, Kim SG, Kim MJ, Lee J, Choi BK, Jin M, et al. Suppressive effect of tomentosin on the production of inflammatory mediators in RAW264.7 cells. Biol Pharm Bull. 2014;37(7):1177–83. DOI: 10.1248/bpb.b14-00050; PMID: 24989009
36. Khaliq H, Ju-Ming Z, Ju-Ming Z, Kemei P. The Physiological Role of Boron on Health. Biol Trace Elem Res. 2018;186(1):31–51. DOI: 10.1007/s12011-018-1284-3; PMID: 29546541
37. Bialek M, Czauderna M, Krajewska KA, Przybylski W. Selected Physiological Effects of Boron Compounds for Animals and Humans. A Review. J Anim Feed Sci. 2019;28(4):307–20. DOI: 10.22358/jafs/114546/2019
38. Dessordi R, Ma N. Boron Action in Bone Health. Rheumatol Orthop Med. 2017;2(1):1-3. DOI: 10.15761/ROM.1000112
39. Kabu M, Uyarlar C, Zarczynska K, Milewska W, Sobiech P. The Role of Boron in Animal Health. J Elem. 2015;20(2):535–41. DOI: 10.5601/jelem.2014.19.3.706
40. Ding Y, Chen Y, Hu K, Li Y, Huang M. Sweroside alleviates hepatic steatosis in part by activating AMPK/mTOR-mediated autophagy in mice. J Cell Biochem. 2023;124(7):1012–22. DOI: 10.1002/jcb.30428; PMID: 37269482
41. Brinza I, El Raey MA, El-Kashak WA, Eldahshan OA, Hritcu L. Sweroside Ameliorated Memory Deficits in Scopolamine-Induced Zebrafish (Danio Rerio) Model: Involvement of Cholinergic System and Brain Oxidative Stress. Molecules. 2022;27(18):5901. DOI: 10.3390/molecules27185901; PMCID: PMC9502219; PMID: 36144637
42. Choi LY, Kim MH, Yang WM. Promotion of osteogenesis by Sweroside via BMP2-involved signaling in postmenopausal osteoporosis. Phytother Res. 2021;35(12):7050-63. DOI: 10.1002/ptr.7336; PMID: 34818696
43. Wang J, Cai XL, Ma R, Lei D, Pan X, Wang F. Anti-inflammatory Effects of Sweroside on LPS-Induced ALI in Mice Via Activating SIRT1. Inflammation. 2021;44(5):1961–8. DOI: 10.1007/s10753-021-01473-4; PMID: 33913051
44. Li J, Zhao C, Zhu Q, Wang Y, Li G, Li X, et al. Sweroside Protects Against Myocardial Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress and Pyroptosis Partially via Modulation of the Keap1/Nrf2 Axis. Front Cardiovasc Med. 2021;8:650368. DOI: 10.3389/fcvm.2021.650368; PMCID: PMC8017130; PMID: 33816579
45. Negi H, Saikia SK, Pandey R. 3β-Hydroxy-urs-12-en-28-oic Acid Modulates Dietary Restriction Mediated Longevity and Ameliorates Toxic Protein Aggregation in C. elegans. J Gerontol A Biol Sci Med Sci. 2017;72(12):1614–9. DOI: 10.1093/gerona/glx118; PMCID: PMC5861981; PMID: 28673026
46. Sharma JK, Bhargava P, Arya D, Bhatia J. 4-Octyl Itaconate Attenuates Isoproterenol Induced Myocardial Damage via NLRP3 Inflammasome Pathway, Nrf-2/HO-1 and MAPK Pathway in an Experimental Model. J Hypertens. 2023;41(Suppl 3):e177. DOI: 10.1097/01.hjh.0000940712.33222.b4
47. Tran DN, Jung EM, Yoo YM, Jeung EB. 4-tert-Octylphenol Exposure Disrupts Brain Development and Subsequent Motor, Cognition, Social, and Behavioral Functions. Oxid Med Cell Longev. 2020;2020:8875604. DOI: 10.1155/2020/8875604; PMCID: PMC7691001; PMID: 33294128
48. Lin JH, Yang KT, Ting PC, Luo YP, Lin DJ, Wang YS, et al. Gossypol Acetic Acid Attenuates Cardiac Ischemia/Reperfusion Injury in Rats Via an Antiferroptotic Mechanism. Biomolecules. 2021;11(11):1667. DOI: 10.3390/biom11111667; PMCID: PMC8615989; PMID: 34827665
49. Hitl M, Kladar N, Gavaric N, Bozin B. Rosmarinic Acid–Human Pharmacokinetics and Health Benefits. Planta Med. 2021;87(4):273–82. DOI: 10.1055/a-1301-8648; PMID: 33285594
50. Mayer M, Berger A, Leischner C, Renner O, Burkard M, Bocker A, et al. Preclinical Efficacy and Toxicity Analysis of The Pan-Histone Deacetylase Inhibitor Gossypol for The Therapy of Colorectal Cancer or Hepatocellular Carcinoma. Pharmaceuticals. 2022;15(4):438. DOI: 10.3390/ph15040438; PMCID: PMC9028974; PMID: 35455435
51. Anidya DK, Purwono RM, Andrianto D, Kusumawati NT. Aktivitas Antibakteri Senyawa Aktif Ekstrak Jintan Hitam (Nigella sativa) Terhadap Bakteri MRSA Secara In Silico. J Vet Biomed. 2023;1(2):92–101. DOI: 10.29244/jvetbiomed.1.2.92-102.
52. Batra G, Anand A, Sharma S, Sharma S, Bhansali S, Patil AN. Scopoletin Improves Glucose Homeostasis in The High-Fructose High-Fat Diet-Induced Diabetes Model in Wistar Rats. J Med Food. 2023; 26(4):270-4. DOI: 10.1089/jmf.2022.k.0153; PMID: 36930782
53. Meilawati L, Dewi R, Tasfiyati AN, Septama AW, Antika LD. Scopoletin: Anticancer Potential and Mechanism of Action. Asian Pacific J Trop Biomed. 2022;13(1):1–8. DOI: 10.4103/2221-1691.367685
54. He B, Liu Z, Li BZ, Yuan YJ. Advances in Biosynthesis of Scopoletin. Microb Cell Fact. 2022;21:152. DOI: 10.1186/s12934-022-01865-7
55. Parama D, Girisa S, Khatoon E, Kumar A, Alqahtani MS, Abbas M, et al. An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacol Res. 2022;179:106202. DOI: 10.1016/j.phrs.2022.106202; PMID: 35378275
56. Lee YJ, Ju CS, Kim IH. Novel Strategy for Synthesis of Stearidonic Acid Enriched Triacylglycerol from Ahiflower Seed Oil Via A Two-Step Enzyme Reaction. In: Proceedings of 2022 AOCS Annual Meeting & Expo. Champaign (IL): American Oil Chemists' Society; 2022. DOI: 10.21748/uhjd7801
57. Li Y, Lai W, Zheng C, Babu JR, Xue C, Ai Q, et al. Neuroprotective Effect of Stearidonic Acid on Amyloid Β-Induced Neurotoxicity in Rat Hippocampal Cells. Antioxidants. 2022;11(12):2357. DOI: 10.3390/antiox11122357; PMCID: PMC9774633; PMID: 36552565
58. Prasad P, Anjali P, Sreedhar RV. Plant-Based Stearidonic Acid as Sustainable Source of Omega-3 Fatty Acid with Functional Outcomes on Human Health. Crit Rev Food Sci Nutr. 2021; 61(10):1725-37. DOI: 10.1080/10408398.2020.1765137; PMID: 32431176
59. Cardoso C, Martinho JP, Lopes P, Martins S, Correia J, Afonso C, et al. Stearidonic acid combined with alpha-linolenic acid improves lipemic and neurological markers in a rat model subject to a hypercaloric diet. Prostaglandins Leukot Essent Fatty Acids. 2018;135:137-46. DOI: 10.1016/j.plefa.2018.07.010; PMID: 30103925
60. Sung J, Jeon H, Kim IH, Jeong HS, Lee J. Anti-Inflammatory Effects of Stearidonic Acid Mediated by Suppression of NF-κB and MAP-Kinase Pathways in Macrophages. Lipids. 2017;52(9):781–7. DOI: 10.1007/s11745-017-4278-6; PMID: 28744771
61. Song MY, Wang JX, Sun YL, Han ZF, Zhou Y, Liu Y, et al. Tetrandrine Alleviates Silicosis by Inhibiting Canonical and Non-Canonical NLRP3 Inflammasome Activation in Lung Macrophages. Acta Pharmacol Sin. 2021;43(5):1274-84. DOI: 10.1038/s41401-021-00693-6; PMCID: PMC9061833; PMID: 34417574
62. Liu T, Li K, Zhang Z, Peng J, Yang J, Law BYK, et al. Tetrandrine Inhibits Cancer Stem Cell Characteristics and Epithelial to Mesenchymal Transition in Triple-Negative Breast Cancer via SOD1/ROS Signaling Pathway. Am J Chin Med. 2023;51(2):425–44. DOI: 10.1142/s0192415x23500222; PMID: 36692485
63. Aditya M, Ariyanti PR. Manfaat Gambir (Uncaria gambir Roxb) sebagai Antioksidan. Majority Med J Lampung Univ. 2016;5(3):129-33.
64. Alfani NR, Febriyanti R, Amananti W. Analysis of Total Flavonoid Content in the Extract of Bajakah Kalalawit Root (Uncaria gambir Roxb) Infunded Results. Indones J Chem Sci Technol. 2023;6(1):65–75. DOI: 10.24114/ijcst.v6i1.43184
65. Indriyah SN, Permatasari DAI, Pratam KJ. Penetapan Kadar Fenolik Serta Uji Aktivitas Antioksidan Ekstrak dan Fraksi Batang Bajakah Kalalawit (Uncaria gambir Roxb) dengan Metode FRAP. Usada Nusantara J Kesehatan Tradisional. 2023;1(2):147–58. DOI: 10.47861/usd.v1i2.347
66. Permatasari S, Halisa N, Frethernety A. Bioactivity Examination of Uncaria gambir (W.Hunter) Roxb on In Vitro Human Sperm Motility. Med Lab Technol J. 2023;9(2):193–201. DOI: 10.31964/mltj.v9i2.563
67. Salsabilla H, Febriyanti R, Amananti W. Penentuan Aktivitas Antioksidan Infudasi Akar Bajakah Tampala (Spatholobus littoralis Hassk) dan Kalalawit (Uncaria gambir Roxb) dengan Metode DPPH. J Cryst Publikasi Penelitian Kimia Terapannya. 2023;5(1):22–9. DOI: 10.36526/jc.v5i1.2583
68. Al Ansori F, Lipoeto NI. Pengaruh Pemberian Kawa Daun Gambir terhadap Kadar Malondialdehid Jaringan Hati Mencit Diabetes yang Diinduksi Aloksan. J Ilmu Kesehatan Indones. 2020;1(1):20–5. DOI: 10.25077/jikesi.v1i1.18
69. Mahfudh N, Thoyyibah RN, Utami D, Nashihah S, Ahda M, Andika A. Screening of antioxidant active fraction of Uncaria gambir Roxb (Bajakah kalawit) wood extract and determination of total phenolics and flavonoids. Afr J Biol Sci. 2024;6(15):11645-57. DOI: 10.48047/AFJBS.6.15.2024.11645-11657
70. Aliviyanti RUY, Sudibyo RS, Murwanti R. Efek Sitotoksik Beberapa Akar Bajakah Kalimantan Terhadap Sel Kanker Payudara T47D. J Penelitian Saintek. 2021;26(2):131–40. DOI: 10.21831/jps.v26i2.41211
71. Yimam M, Lee YC, Kim TW, Moore B, Jiao P, Hong M, et al. Analgesic and anti-inflammatory effect of UP3005, a botanical composition containing two standardized extracts of Uncaria gambir and Morus alba. Pharmacogn Res. 2015;7(Suppl 1):S39-46. DOI: 10.4103/0974-8490.157995; PMCID: PMC4466767; PMID: 26109786
72. Oswari L, Hidayat R, Fatmawati F, Hayati L, Alisa BS. Gambir Extract (Uncaria Gambir) Decreases Inflammatory Response and Increases Gastric Mucosal Integrity in Wistar Rats - Model Gastritis. Open Access Maced J Med Sci. 2019;7(19):3149-52. DOI: 10.3889/oamjms.2019.758; PMCID: PMC6953953; PMID: 31949507
73. Yunarto N, Intan PR, Kurniatri AA, Sulistyowati I, Aini N. Anti-Inflammatory Activities of Ethyl Acetate Fraction from Uncaria gambir Leaves Through the Inhibition of Edema, COX-2 and iNOS Expression. In: Permatasari TAE, Endarti AT, Kusuma D, Muhidin S, Widiatmoko D, Ramadhan AI, editors. Proceedings of the 4th International Symposium on Health Research (ISHR 2019). Paris: Atlantis Press; 2020. p. 108–12. DOI: 10.2991/ahsr.k.200215.021
74. Syarifah S, Widyawati T, Anggraini DR, Wahyuni AS, Sari MI. Anticancer activity of Uncaria gambir roxb on T47D breast cancer cells. J Phys Conf Ser. 2019;1317:012106. DOI: 10.1088/1742-6596/1317/1/012106
75. Malrianti Y, Kasim A, Asben A, Syafri E, Yeni G, Fudholi A. Catechin Extracted from Uncaria gambier Roxb for Nanocatechin Production: Physical and Chemical Properties. Int J Des Nat Ecodynamics. 2021;16(4):393-9. DOI: 10.18280/ijdne.160406
76. Rahmawati N, Fernando A. Kandungan Fenolik dan Aktivitas Antioksidan Ekstrak Daun Gambir Kering (Uncaria gambir (Hunter) Roxb). Indones Chem Acta. 2013;4(1):1–6.
77. Sazwi NN, Nalina T, Rahim ZHA. Antioxidant and cytoprotective activities of Piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complement Altern Med. 2013;13:351. DOI: 10.1186/1472-6882-13-351; PMCID: PMC4029269; PMID: 24330738
78. Widiyarti G, Sundowo A, Filailla E, Laksmono JA. The Mechanically Extraction Process of Gambier (Uncaria gambier Roxb.) from Limapuluh Kota,West Sumatera and Its Antioxidant activity. J Pure Appl Chem Res. 2020;9(1):8-15. DOI: 10.21776/jpacr.ub.2020.009.01.509
79. Yanti E, Morika HD, Harmawati, Nur SA. Pengaruh Pemberian Gambir (Uncaria Gambir) Terhadap Kadar Gula Darah Pada Pasien Diabetes Melitus Tipe II. J Kesehatan Saintika Meditory. 2020;2(2):27–39. DOI: 10.30633/jsm.v2i2.543
80. Widiyarti G, Sundowo A, Hanafi M. The Free Radical Scavenging and Anti-Hyperglycemic Activities of Various Gambiers Available in Indonesian Market. Makara J Sci. 2011;15(2):22.
81. Apea-Bah FB, Hanafi M, Dewi RT, Fajriah S, Darwaman A, Artanti N, et al. Assessment of the DPPH and-glucosidase inhibitory potential of gambier and qualitative identification of major bioactive compound. J Med Plant Res. 2009;3(10):736-57.
82. Zebua EA, Silalahi J, Julianti E. Hypoglicemic Activity of Gambier (Uncaria gambir Roxb.) Drinks in Alloxan-Induced Mice. IOP Conf Ser Earth Environ Sci. 2018;122:012088. DOI: 10.1088/1755-1315/122/1/012088
83. Ningsih S, Fachrudin F, Rismana E, Purwaningsih EH, Sumaryono W, Jusman SWA. Evaluation of antilipid peroxidation activity of Gambir extract on liver homogenat in vitro. Int J Pharmtech Res. 2014;6(3):982–9.
84. Rismana E, Ningsih S, Fachrudin F. In vitro study of xanthine oxidase inhibitory of gambir (Uncaria gambir) hunter roxb extracts. Pharmacogn J. 2017;9(6):862-5. DOI: 10.5530/pj.2017.6.135
Authors
Copyright (c) 2025 Ainun Fawaid, Nurkhasanah Mahfudh

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.