Discovery of Novel Alkaloids from Magnolia Genus: A Literature Review from 2002-2024
Abstract
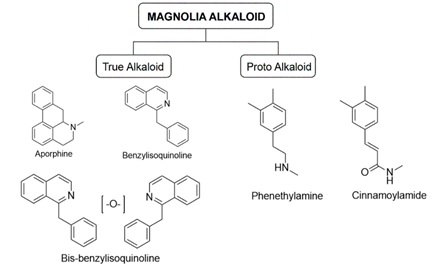
The genus Magnolia, encompassing hundreds of globally distributed species, has a long-standing history in traditional medicine for treating diverse ailments. These species are particularly renowned for their rich array of bioactive compounds, notably alkaloids. This study provides a comprehensive summary of novel alkaloid compounds identified in various Magnolia species within recent years. Through a targeted literature review utilizing Google Scholar and PubMed (2002–2024), we pinpointed nine novel alkaloids and one nitrogen-based compound isolated from four distinct Magnolia species. These newly discovered compounds exhibited promising bioactivities, including significant antiplatelet and anti-acetylcholinesterase effects. Structurally, the majority of these compounds belong to the aporphine and benzylisoquinoline classes, although some display unique configurations, such as glycosidic or N-oxide alkaloids. This review aims to bridge a critical gap in the existing scientific literature regarding the comprehensive documentation of novel alkaloid secondary metabolites found across the diverse Magnolia genus.
Full text article
References
2. Yahaya AAH, Salleh WMNHW, Ghani NA. Magnolia genus - A systematic review on the composition and biological properties of its essential oils. Riv Ital Sostanze Grasse. 2022;99(3):249-61.
3. Shi S, Zhong Y, Hoch WA. Distribution and Commercial Cultivation of Magnolia. In: Sarker SD, Maruyama Y, editors. Magnolia: The Genus Magnolia. London: Taylor & Francis; 2002.
4. Kinoshita T. A supplement to Prof. Shoji Shibata’s achievements: the history of “Shōsō-in Medicines” and the reason why Magnolia obovata (old name: Hoogashiwa) was not given a Chinese herbal name in Japan’s oldest anthology “Man’yōshū”. J Nat Med. 2018;72:2-11. DOI: 10.1007/s11418-017-1112-7; PMID: 28748433
5. Domínguez-Yescas R, Vázquez-García JA. Flower of the heart, Magnolia yajlachhi (subsect. Talauma, Magnoliaceae), a new species of ceremonial, medicinal, conservation and nurse tree relevance in the Zapotec culture, Sierra Norte de Oaxaca, Mexico. Phytotaxa. 2019;393(1):21-34. DOI: 10.11646/phytotaxa.393.1.2
6. Darma IDP, Sutomo S, Hanum SF, Iryadi R, Rahayu A. Flowers and Value of Conservation in The Culture of Hindu Community in Bali. Biosaintifika Berkala Ilmiah Biol. 2021;13(1):34-40. DOI: 10.15294/biosaintifika.v13i1.27054
7. Kulak V, Longboat S, Brunet ND, Shukla M, Saxena P. In Vitro Technology in Plant Conservation: Relevance to Biocultural Diversity. Plants. 2022;11(4):503. DOI: 10.3390/plants11040503; PMCID: PMC8876341; PMID: 35214833
8. Pironon S, Ondo I, Diazgranados M, Allkin R, Baquero AC, Cámara-Leret R, et al. The global distribution of plants used by humans. Science. 2024;383(6680):293-7. DOI: 10.1126/science.adg8028; PMID: 38236975
9. Poivre M, Duez P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J Zhejiang Univ Sci B. 2017;18(3):194-214. DOI: 10.1631/jzus.B1600299; PMCID: PMC5365644; PMID: 28271656
10. Vázquez-García JA, Navas ET, Archila F, Véliz-Pérez ME. Magnolia ottoi (Magnoliaceae) a new species from Purulhá, Baja Verapaz, Guatemala: Conservation and Mayan Q'eqchi 'ethnotaxonomy. Phytotaxa. 2020;455(3):187-95. DOI: 10.11646/phytotaxa.455.3.1
11. Kelm MA, Nair MG. A Brief Summary of Biologically Active Compounds from Magnolia spp. Stud Nat Prod Chem. 2000;24(E):845-73. DOI: 10.1016/S1572-5995(00)80056-3
12. Heinrich M, Mah J, Amirkia V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity-An Update and Forward Look. Molecules. 2021;26(7):1836. DOI: 10.3390/molecules26071836; PMCID: PMC8036335; PMID: 33805869
13. Lin GD, Vishwakarma P, Smith PN, Li RW. The Occurrence and Bioactivities of Amaryllidaceae Alkaloids from Plants: A Taxonomy-Guided Genera-Wide Review. Plants. 2025;14(13):1935. DOI: 10.3390/plants14131935; PMCID: PMC12252167; PMID: 40647943
14. Abookleesh FL, Al-Anzi BS, Ullah A. Potential Antiviral Action of Alkaloids. Molecules. 2022;27(3):903. DOI: 10.3390/molecules27030903; PMCID: PMC8839337; PMID: 35164173
15. Bhambhani S, Kondhare KR, Giri AP. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules. 2021;26(11):3374. DOI: 10.3390/molecules26113374; PMCID: PMC8199754; PMID: 34204857
16. Morris JS, Facchini PJ. Isolation and Characterization of Reticuline N-Methyltransferase Involved in Biosynthesis of the Aporphine Alkaloid Magnoflorine in Opium Poppy. J Biol Chem. 2016;291(45):23416-27. DOI: 10.1074/jbc.m116.750893; PMCID: PMC5095398; PMID: 27634038
17. Hagel JM, Facchini PJ. Benzylisoquinoline Alkaloid Metabolism: A Century of Discovery and a Brave New World. Plant Cell Physiol. 2013;54(5):647-72. DOI: 10.1093/pcp/pct020; PMID: 23385146
18. Yan R, Wang W, Guo J, Liu H, Zhang J, Yang B. Studies on the alkaloids of the bark of Magnolia officinalis: isolation and on-line analysis by HPLC-ESI-MS(n). Molecules. 2013;18(7):7739-50. DOI: 10.3390/molecules18077739; PMCID: PMC6270518; PMID: 23823874
19. Zhu R, Jiang G, Tang W, Zhao W, Chen F, Zhang X, et al. Aporphines: A privileged scaffold in CNS drug discovery. Eur J Med Chem. 2023;256:115414. DOI: 10.1016/j.ejmech.2023.115414; PMID: 37172474
20. Sun J, Zhan X, Wang W, Yang X, Liu Y, Yang H, et al. Natural aporphine alkaloids: A comprehensive review of phytochemistry, pharmacokinetics, anticancer activities, and clinical application. J Adv Res. 2024;63:231-53. DOI: 10.1016/j.jare.2023.11.003; PMCID: PMC11380034; PMID: 37935346
21. Weber C, Opatz T. Bisbenzylisoqinoline Alkaloids. Alkaloids Chem Biol. 2019;81:1-114. DOI: 10.1016/bs.alkal.2018.07.001; PMID: 30685048
22. Lichman BR. The scaffold-forming steps of plant alkaloid biosynthesis. Nat Prod Rep. 2021;38(1):103-29. DOI: 10.1039/D0NP00031K
23. Kawahara T, Tomono T, Hamauzu Y, Tanaka K, Yasui H. Inhibitory Effect of a Hot-Water Extract of Leaves of Japanese Big-Leaf Magnolia (Magnolia obovata) on Rotavirus-Induced Diarrhea in Mouse Pups. Evid Based Complement Alternat Med. 2014;2014:365831. DOI: 10.1155/2014/365831; PMCID: PMC4279284; PMID: 25580150
24. Pyo MK, Yun-Choi HS, Hong YJ. Antiplatelet Activities of Aporphine Alkaloids Isolated from Leaves of Magnolia obovata. Planta Med. 2003;69(3):267-9. DOI: 10.1055/s-2003-38493; PMID: 12677533
25. Nonato MG, Garson MJ, Truscott RJW, Carver JA. 1H-NMR Assignments of Anonaine and Xylopine Derivatives from Talauma gitingensis. J Nat Prod. 1990;53(6):1623-7. DOI: 10.1021/np50072a043
26. Fiagbe NI, Lin FT, Lin MC, Aly Y, Schiff Jr PL. Alkaloids of Hexalobus monopetalus. Planta Med. 1988;54(2):177. DOI: 10.1055/s-2006-962386; PMID: 17265237
27. Pachaly P, Adnan AZ, Will G. N-Formyl- and N-Acetyl-Aporphine Alkaloids of Tinospora crispa. Planta Med. 1989;55(1):115-6. DOI: 10.1055/s-2006-961899
28. Mori LS, Boller S, Kassuya CAL, Stefanello MEA, Zampronio AR. Analgesic effects of the ethanolic extract from Magnolia ovata (Magnoliaceae) trunk bark and of N-acetylxylopine, a semi-synthetic analogue of xylopine. Phytomedicine. 2011;18(2-3):143-7. DOI: 10.1016/j.phymed.2010.06.001; PMID: 20637574
29. Wang HM, Chen CY, Chen CY, Ho ML, Chou YT, Chang HC, et al. (−)-N-Formylanonaine from Michelia alba as a human tyrosinase inhibitor and antioxidant. Bioorg Med Chem. 2010;18(14):5241-7. DOI: 10.1016/j.bmc.2010.05.045; PMID: 20584613
30. Tsakadze DM, Samsoniya SA, Ziaev R, Abdusamatov A. Alkaloid and phenolic compounds of Galanthus caucasicus, Magnolia obovata, Cocculus laurifolius, and Veratrum lobelianum grown in Georgia. Mol Divers. 2005;9(1-3):41-4. DOI: 10.1007/s11030-005-2100-5; PMID: 15789550
31. Yang X, Miao X, Dai L, Guo X, Jenis J, Zhang J, et al. Isolation, biological activity, and synthesis of isoquinoline alkaloids. Nat Prod Rep. 2024;41(11):1652-722. DOI: 10.1039/d4np00023d; PMID: 39355982
32. Rivers M, Beech E, Murphy L, Oldfield S. The Red List of Magnolaiceae: Revised and Extended. Richmond, Surrey: Botanic Gardens Conservation International;2016.
33. Chang HS, Cheng MJ, Chen IS. Secondary Metabolites from Magnolia kachirachirai. Helv Chim Acta. 2011;9(4):703-10. DOI: 10.1002/hlca.201000289
34. Dashti Y, Hobson C, Tajabadi FM, Rezadoost H. Phytochemical Investigation of Iphiona aucheri. Structural Revision of Donine. Chem Nat Compd. 2019;55:902-7. DOI: 10.1007/s10600-019-02842-0
35. Song C, Liu H, Gao J. Habitat preference and potential distribution of Magnolia officinalis subsp. officinalis and M. o. subsp. biloba in China. Nat Conserv. 2019;36:93-111. DOI: 10.3897/natureconservation.36.36171
36. Aziz DM, Taher SG, Hama JR. Isolation and identification of new alkaloids from purslane (Portulaca oleracea l.) leaves using HPLC/ESIMS. MOJ Food Process Technol. 2016;2(4):148-51. DOI: 10.15406/mojfpt.2016.02.00047
37. Guo ZF, Wang XB, Luo JG, Luo J, Wang JS, Kong LY. A novel aporphine alkaloid from Magnolia officinalis. Fitoterapia. 2011;82(4):637-41. DOI: 10.1016/j.fitote.2011.01.021; PMID: 21291961
38. Yu Y, Du Y, Dong J, Yin Z, Chen P, Cao L, et al. Phytotoxic Effects of the Aqueous Extracts of Magnolia biondii Pamp. Flower Litter and the Joint Action of Allelochemicals. Plants. 2025;14(11):1577. DOI: 10.3390/plants14111577; PMCID: PMC12158015; PMID: 40508252
39. Cao Y, Li H, Zhang Y, Wang J, Ren Y, Liu Y, et al. Alkaloids and lignans with acetylcholinesterase inhibitory activity from the flower buds of Magnolia biondii Pamp. New J Chem. 2020;44(25):10309-16. DOI: 10.1039/D0NJ01537G
40. Martin-Tanguy J. The occurrence and possible function of hydroxycinnamoyl acid amides in plants. Plant Growth Regul. 1985;3:381-99. DOI: 10.1007/bf00117595
41. Okano M, Sato M, Kageyama S. Determination of higenamine and coclaurine levels in human urine after the administration of a throat lozenge containing Nandina domestica fruit. Drug Test Anal. 2017;9(11-12):1788-93. DOI: 10.1002/dta.2258
42. Tian W, Zhi H, Yang C, Wang L, Long J, Xiao L, et al. Chemical composition of alkaloids of Plumula nelumbinis and their antioxidant activity from different habitats in China. Ind Crop Prod. 2018;125:537-48. DOI: 10.1016/j.indcrop.2018.09.045
Authors
Copyright (c) 2025 Tegar Achsendo Yuniarta, Rosita Handayani

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.