Antibiotic-Resistant Profiles of Bacteria Isolated from Cesarean and Surgical Patients from Kasese District Hospitals Western Uganda
Abstract
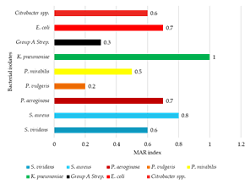
Surgical site infections (SSIs) are challenging to treat and often associated with much higher extended stays, morbidity, and mortality, higher treatment costs, especially when the causative agent is multidrug resistance (MDR). This study was designed to determine the prevalence of nosocomial infections and susceptibility profiles of bacteria isolated from Cesarean section (C-section) and surgical patients from Kasese District Hospitals in Western Uganda. A descriptive cross-sectional study was conducted from January to September 2016 involving 303 patients with SSIs in obstetrics & gynecology; and general surgery wards in three health facilities. Clinical-demographic characteristics of patients were obtained using structured questionnaires before surgery. Bacterial analysis of the air and floor of the theatre room was done using the standard culture method. Of the 303 patients enrolled with SSIs (median age 34 years), 71.6% were female, and 28.4% were males. Only 14.5% developed SSIs, with predominant isolates being Staphylococcus aureus 33.33% and Escherichia coli 24%. The majority of recruited participants underwent a C-section of 58% and the least amputations of 0.3%. Duration of operation or surgery, p-value 0.002 (95% CI 1.599-7.667) was significantly associated with SSIs. Gram-negative bacteria were found resistant (50-100%) to ampicillin, gentamycin, and ciprofloxacin, the commonly used post-operative drugs of choice. Hospital-acquired infections were common with emerging antibiotic-resistant strains isolated in most SSIs at Kasese hospitals. The development of resistance to commonly used antibiotics such as ampicillin, gentamycin, and ciprofloxacin than previously reported calls for laboratory-guided SSIs therapy and strengthening infection control policies.
Full text article
References
2. Khan HA, Baig FK, Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478-82. doi:10.1016/j.apjtb.2017.01.019
3. Voidazan S, Albu S, Toth R, Grigorescu B, Rachita A, Moldovan I. Healthcare Associated Infections—A New Pathology in Medical Practice? Int J Environ Res Public Health. 2020;17(3):760. doi:10.3390/ijerph17030760
4. Revelas A. Healthcare – associated infections: A public health problem. Niger Med J. 2012;53(2):59-64. doi:10.4103/0300-1652.103543
5. Adam AS, Micheni L, Onkoba SK, Ntulume I, Aliero AA, Namatovu A. Antibiotic Susceptibility Pattern and Detection of mecA Gene in Methicillin Resistant Staphylococcus Epidermidis Isolated from Wards Surfaces of Kampala International University Teaching Hospital, Uganda. Rom Arch Microbiol Immunol. 2020;79(1):24-36.
6. Azeez-Akande O. Emerging and re-emerging infectious agents of nosocomial diseases – The need for review of hospital policy and control strategies. Bayero J Pure Appl Sci. 2012;5(2):19-25. doi:10.4314/bajopas.v5i2.3
7. Ssekitoleko RT, Oshabaheebwa S, Munabi IG, Tusabe MS, Namayega C, Ngabirano BA, et al. The role of medical equipment in the spread of nosocomial infections: a cross-sectional study in four tertiary public health facilities in Uganda. BMC Public Health. 2020;20(1):1561. doi:10.1186/s12889-020-09662-w
8. Seni J, Najjuka CF, Kateete DP, Makobore P, Joloba ML, Kajumbula H, et al. Antimicrobial resistance in hospitalized surgical patients: a silently emerging public health concern in Uganda. BMC Res Notes. 2013;6:298. doi:10.1186/1756-0500-6-298
9. Tolera M, Marami D, Abate D, Dheresa M. Are Invasive Procedures and a Longer Hospital Stay Increasing the Risk of Healthcare-Associated Infections among the Admitted Patients at Hiwot Fana Specialized University Hospital, Eastern Ethiopia? Adv Prev Med. 2020;2020:6875463. doi:10.1155/2020/6875463
10. Danasekaran R, Mani G, Annadurai K. Prevention of healthcare-associated infections: protecting patients, saving lives. Int J Community Med Public Health. 2014;1(1):67-8. doi:10.5455/2394-6040.ijcmph20141114
11. Abadi ATB, Rizvanov AA, Haertlé T, Blatt TL. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience. 2019;9:778-88. doi:10.1007/s12668-019-00658-4
12. Agaba P, Tumukunde J, Tindimwebwa JVB, Kwizera A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: a cross sectional study. BMC Res Notes. 2017;10(1):349. doi:10.1186/s13104-017-2695-5
13. Wasswa P, Nalwadda CK, Buregyeya E, Gitta SN, Anguzu P, Nuwaha F. Implementation of infection control in health facilities in Arua district, Uganda: a cross-sectional study. BMC Infect Dis. 2015;15:268. doi:10.1186/s12879-015-0999-4
14. Greco D, Magombe I. Hospital acquired infections in a large north Ugandan hospital. J Prev Med Hyg. 2011;52(2):55-8.
15. Omair A. Sample size estimation and sampling techniques for selecting a representative sample. J Health Specialties. 2014;2(4):142-7. doi:10.4103/1658-600X.142783
16. Hodges AM, Agaba S. Wound infection in a rural hospital: the benefit of a wound management protocol. Trop Doct. 1997;27(3):174-5. doi:10.1177/004947559702700321
17. Getachew H, Derbie A, Mekonnen D. Surfaces and Air Bacteriology of Selected Wards at a Referral Hospital, Northwest Ethiopia: A Cross-Sectional Study. Int J Microbiol. 2018;2018:6413179. doi:10.1155/2018/6413179
18. Najotra DK, Malhotra AS, Slathia P, Raina S, Dhar A. Microbiological Surveillance of Operation Theatres: Five Year Retrospective Analysis from a Tertiary Care Hospital in North India. Int J Appl Basic Med Res. 2017;7(3):165-8. doi:10.4103/ijabmr.ijabmr_281_16
19. Onyekwelu I, Yakkanti R, Protzer L, Pinkston CM, Tucker C, Seligson D. Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient. J Am Acad Orthop Surg Glob Res Rev. 2017;1(3):e022. doi:10.5435/JAAOSGlobal-D-17-00022
20. Iskender NA, Algur OF, Aksu Y, Saral A. Isolation, identification and characterization of biotechnologically important bacteria from microflora of Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae). Biotechnol Biotechnol Equip. 2017;31(3):505-10. doi:10.1080/13102818.2017.1294035
21. Balouri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71-9. doi:10.1016/j.jpha.2015.11.005
22. Khan ZA, Siddiqui MF, Park S. Current and Emerging Methods of Antibiotic Susceptibility Testing. Diagnostics. 2019;9(2):49. doi:10.3390/diagnostics9020049
23. Ayandele AA, Oladipo EK, Oyebisi O, Kaka MO. Prevalence of Multi-Antibiotic Resistant Escherichia coli and Klebsiella species obtained from a Tertiary Medical Institution in Oyo State, Nigeria. Qatar Med J. 2020;2020(1):9. doi:10.5339/qmj.2020.9
24. Osundiya OO, Oladele RO, Oduyebo OO. Multiple Antibiotic Resistance (MAR) indices of Pseudomonas and Klebsiella species isolates in Lagos University Teaching Hospital. African J Clin Exp Microbiol. 2013;14(3):164-8. doi:10.4314/ajcem.v14i3.8
25. Nair A, Steinberg WJ, Habib T, Saeed H, Raubenheimer JE. Prevalence of healthcare-associated infection at a tertiary hospital in the Northern Cape Province, South Africa. S Afr Fam Pract. 2018;60(5):162-7. doi:10.1080/20786190.2018.1487211
26. Yallew WW, Kumie A, Yehuala FM. Point prevalence of hospital-acquired infections in two teaching hospitals of Amhara region in Ethiopia. Drug Healthc Patient Saf. 2016;8:71-6. doi:10.2147/dhps.s107344
27. Okello TR, Kansiime J, Odora J. Invasive procedures and Hospital Acquired Infection (HAI) in A large hospital in Northern Uganda. East Cent Afr J Surg. 2014;19(3):77-84.
28. Kesah CNF, Egri-Okwaji MTC, Iroha E, Odugbemi TO. Aerobic bacterial nosocomial infections in paediatric surgical patients at a tertiary health institution in Lagos, Nigeria. Niger Postgrad Med J. 2004;11(1):4-9.
29. Tang CS, Wan GH. Air quality monitoring of the post-operative recovery room and locations surrounding operating theaters in a medical center in Taiwan. PLoS One. 2013;8(4):e61093. doi:10.1371/journal.pone.0061093
30. Onwubiko NE, Ejike N, Onyinyechi NP. Microbial contamination of air and protective wears in the operating theatre and surgical wards of two tertiary hospitals in Kano, Northwestern Nigeria. Int J Infect Control. 2014;11(3):3. doi:10.3396/IJIC.v11i3.020.15
31. Genet C, Kibru G, Tsegaye W. Indoor Air Bacterial Load and Antibiotic Susceptibility Pattern of Isolates in Operating Rooms and Surgical Wards at Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci. 2011;21(1):9-17. doi:10.4314/ejhs.v21i1.69039
32. Al Laham NA. Prevalence of bacterial contamination in general operating theaters in selected hospitals in the Gaza Strip, Palestine. J Infect Public Health. 2012;5(1):43-51. doi:10.1016/j.jiph.2011.10.006
33. Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016;4(2):1-24. doi:10.1128/microbiolspec.VMBF-0016-2015
34. Yimenu DK, Emam A, Elemineh E, Atalay W. Assessment of Antibiotic Prescribing Patterns at Outpatient Pharmacy Using World Health Organization Prescribing Indicators. I Prim Care Community Health. 2019;10: 2150132719886942. doi:10.1177/2150132719886942
35. Stanley IJ, Kajumbula H, Bazira J, Kansiime C, Rwego IB, Asiimwe BB. Multidrug resistance among Escherichia coli and Klebsiella pneumoniae carried in the gut of out-patients from pastoralist communities of Kasese district, Uganda. PLoS One. 2018;13(7):e0200093. doi:10.1371/journal.pone.0200093
Authors
Copyright (c) 2021 Abraham Bwalhuma Muhindo, Adamu Almustapha Aliero, Martin Odoki, Ibrahim Ntulume, Emmanuel Eilu, Joe Mutebi, Yap Boum II, Richard Onyuthi Apecu

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.