Monoclonal Antibodies: A Therapeutic Option for the Treatment of Ophthalmic Diseases of the Eye Posterior Segment
Abstract
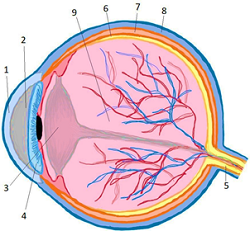
The eye is an organ that allows us to observe the outside world. Pathologies of the eye's posterior segment, such as glaucoma, macular degeneration, diabetic retinopathy, uveitis, and retinoblastoma, cause vision loss. Traditional treatments consist of applying topical medications that do not penetrate properly or using high doses that generate adverse effects. Different laser surgeries stop the pathology's progression but do not allow visual improvement. So, an alternative is to use monoclonal antibodies, proteins produced by different processes that selectively bind to metabolites associated with diseases, reducing the adverse effects of traditional treatments and improving the application of the drug in the area. The two main molecular targets are TNF (adalimumab, infliximab, and certolizumab pegol) and VEGF (bevacizumab and ranibizumab); other possibilities are under investigation.
Full text article
References
2. Lankford CK, Laird JG, Inamdar SM, Baker SA. A Comparison of the Primary Sensory Neurons Used in Olfaction and Vision. Front Cell Neurosci. 2020;14:595523. doi:10.3389/fncel.2020.595523
3. Moschos MM. Physiology and psychology of vision and its disorders: a review. Med Hypothesis Discov Innov Ophthalmol. 2014;3(3):83-90.
4. Wentzel A, Mchiza ZJR. Exploring Factors Associated with Diabetic Retinopathy Treatment Compliance Behaviour in Cape Town, South Africa. Int J Environ Res Public Health. 2021;18(22):12209. doi:10.3390/ijerph182212209
5. Demmin DL, Silverstein SM. Visual Impairment and Mental Health: Unmet Needs and Treatment Options. Clin Ophthalmol. 2020;14:4229-51. doi:10.2147/opth.s258783
6. Rai BB, Morley MG, Bernstein PS, Maddess T. Pattern of vitreo-retinal diseases at the national referral hospital in Bhutan: a retrospective, hospital-based study. BMC Ophthalmol. 2020;20(1):51. doi:10.1186/s12886-020-01335-x
7. GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-60. doi:10.1016/s2214-109x(20)30489-7
8. Hart CT, Zhu EY, Crock C, Rogers SL, Lim LL. 2019. Epidemiology of uveitis in urban Australia. Clin Exp Ophthalmol. 47(6):733-40. doi:10.1111/ceo.13517
9. Fabian ID, Sagoo MS. 2018. Understanding retinoblastoma: epidemiology and genetics. Community Eye Health. 31(101):7.
10. Awwad S, Ahmed AHAM, Sharma G, Heng JS, Khaw PT, Brocchini S, et al. Principles of pharmacology in the eye. Br J Pharmacol. 2017;174(23):4205-23. doi:10.1111/bph.14024
11. Gamalero L, Simonini G, Ferrara G, Polizzi S, Giani T, Cimaz R. Evidence-Based Treatment for Uveitis. Isr Med Assoc J. 2019;21(7):475-9.
12. Conlon R, Saheb H, Ahmed IIK. Glaucoma treatment trends: a review. Can J Ophthalmol. 2017;52(1):114-24. doi:10.1016/j.jcjo.2016.07.013
13. Quinteros DA, Bermúdez JM, Ravetti S, Cid A, Allemandi DA, Palma SD. Therapeutic use of monoclonal antibodies: general aspects and challenges for drug delivery. Nanostruct Drug Deliv. 2017:807-33. doi:10.1016/B978-0-323-46143-6.00025-7
14. Mousa SA, Mousa SS. Current status of vascular endothelial growth factor inhibition in age-related macular degeneration. BioDrugs. 2010;24(3):183-94. doi:10.2165/11318550-000000000-00000
15. Awwad S, Angkawinitwong U. Overview of Antibody Drug Delivery. Pharmaceutics. 2018;10(3):83. doi:10.3390/pharmaceutics10030083
16. Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1. doi:10.1186/s12929-019-0592-z
17. Turvey TA, Golden BA. Orbital anatomy for the surgeon. Oral Maxillofac Surg Clin North Am. 2012;24(4):525-36. doi:10.1016/j.coms.2012.08.003
18. de Andrade FA, Fiorot SHS, Benchimol EI, Provenzano J, Martins VJ, Levy RA. The autoimmune diseases of the eyes. Autoimmun Rev. 2016;15(3):258-71. doi:10.1016/j.autrev.2015.12.001
19. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190-4. doi:10.4103/ijo.ijo_646_17
20. Ljubimov AV. Diabetic complications in the cornea. Vision Res. 2017;139:138-52. doi:10.1016/j.visres.2017.03.002
21. Wisely CE, Sayed JA, Tamez H, Zelinka C, Abdel-Rahman MH, Fischer AJ, et al. The chick eye in vision research: An excellent model for the study of ocular disease. Prog Retin Eye Res. 2017;61:72-97. doi:10.1016/j.preteyeres.2017.06.004
22. Rutkowski P, May CA. Nutrition and Vascular Supply of Retinal Ganglion Cells during Human Development. Front Neurol. 2016;7:49. doi:10.3389/fneur.2016.00049
23. Ptito M, Bleau M, Bouskila J. The Retina: A Window into the Brain. Cells. 2021;10(12):3269. doi:10.3390/cells10123269
24. Baino F, Kargozar S. Regulation of the Ocular Cell/Tissue Response by Implantable Biomaterials and Drug Delivery Systems. Bioengineering. 2020;7(3):65. doi:10.3390/bioengineering7030065
25. Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous Humor Dynamics: A Review. Open Ophthalmol J. 2010;4:52-9. doi:10.2174/1874364101004010052
26. Ankamah E, Sebag J, Ng E, Nolan JM. Vitreous Antioxidants, Degeneration, and Vitreo-Retinopathy: Exploring the Links. Antioxidants. 2019;9(1):7. doi:10.3390/antiox9010007
27. Dunn JP. Uveitis. Prim Care. 2015;42(3):305-23. doi:10.1016/j.pop.2015.05.003
28. Listing M, Mönkemöller K, Liedmann I, Niewerth M, Sengler C, Listing J, et al. The majority of patients with newly diagnosed juvenile idiopathic arthritis achieve a health-related quality of life that is similar to that of healthy peers: results of the German multicenter inception cohort (ICON). Arthritis Res Ther. 2018;20(1):106. doi:10.1186/s13075-018-1588-x
29. Angeles-Han ST, Rabinovich CE. Uveitis in children. Curr Opin Rheumatol. 2016;28(5):544–9. doi:10.1097/BOR.0000000000000316
30. Townsend WM. Canine and feline uveitis. Vet Clin North Am Small Anim Pract. 2008;38(2):323-46. doi:10.1016/j.cvsm.2007.12.004
31. Ramstein J, Broos CE, Simpson LJ, Ansel KM, Sun SA, Ho ME, et al. IFN-γ-Producing T-Helper 17.1 Cells Are Increased in Sarcoidosis and Are More Prevalent than T-Helper Type 1 Cells. Am J Respir Crit Care Med. 2016;193(11):1281-91. doi:10.1164/rccm.201507-1499oc
32. Harthan JS, Opitz DL, Fromstein SR, Morettin CE. Diagnosis and treatment of anterior uveitis: optometric management. Clin Optom. 2016;8:23-35. doi:10.2147/opto.s72079
33. Budny A, Grochowski C. Retinoblastoma. J Educ Health Sport. 2018;8(7):204-13. doi:10.5281/zenodo.1299573
34. Rodriguez-Galindo C, Orbach DB, VanderVeen D. Retinoblastoma. Pediatr Clin North Am. 2005;62(1):201-23. doi:10.1016/j.pcl.2014.09.014
35. Ortiz MV, Dunkel IJ. Retinoblastoma. J Child Neurol. 2016;31(2):227-36. doi:10.1177/0883073815587943
36. del Río NR, Gómez JMA, de la Rosa FJAG, Calvo JMP, Martín AdlH. Retinoblastoma trilateral. Correlación de las alteraciones genéticas del gen RB1 y la presencia de quistes en la glándula pineal. Arch Soc Esp Oftalmol. 2014;89(1):4-9. doi:10.1016/j.oftal.2013.07.006
37. Balmer A, Munier F. Differential diagnosis of leukocoria and strabismus, first presenting signs of retinoblastoma. Clin Ophthalmol. 2007;1(4):431-9.
38. ud Din J, Khan Z, Khan I. Diabetic Retinopathy; Prevalence of Diabetic Retinopathy in Recently Diagnosed Type 2 Diabetic Patients. A Single Center Study. Professional Med J. 2019;26(4):663-8. doi:10.29309/TPMJ/2019.26.04.3374
39. Hendrick AM, Gibson MV, Kulshreshtha A. Diabetic Retinopathy. Prim Care. 2015;42(3):451-64. doi:10.1016/j.pop.2015.05.005
40. Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20(26):3218-25. doi:10.2174/09298673113209990022
41. Homme RP, Singh M, Majumder A, George AK, Nair K, Sandhu HS, et al. Remodeling of Retinal Architecture in Diabetic Retinopathy: Disruption of Ocular Physiology and Visual Functions by Inflammatory Gene Products and Pyroptosis. Front Physiol. 2018;9:1268. doi:10.3389/fphys.2018.01268
42. Mehta S. Age-Related Macular Degeneration. 2015. Prim Care. 2015;42(3):377-91. doi:10.1016/S0140-6736(12)60282-7
43. Grassmann F, Fleckenstein M, Chew EY, Strunz T, Schmitz-Valckenberg S, Göbel AP, et al. Clinical and Genetic Factors Associated with Progression of Geographic Atrophy Lesions in Age-Related Macular Degeneration. PloS One. 2015;10(5):0126636. doi:10.1371/journal.pone.0126636
44. Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147-59. doi:10.1016/S0140-6736(18)31550-2
45. Hohenstein-Blaul NVTU, Bell K, Pfeiffer N, Grus FH. Autoimmune aspects in glaucoma. Eur J Pharmacol. 2016;787:105-18. doi:10.1016/j.ejphar.2016.04.031
46. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183-93. doi:10.1016/S0140-6736(17)31469-1
47. Gupta D, Chen PP. Glaucoma. Am Fam Physician. 2016;93(8):668-74.
48. Merino AG. Anticuerpos monoclonales. Aspectos básicos. Neurología. Neurología. 2011;26(5):301-6. doi:10.1016/j.nrl.2010.10.005
49. Bertelsen MB, Senissar M, Nielsen MH, Bisiak F, Cunha MV, Molinaro AL, Daines DA, et al. Structural Basis for Toxin Inhibition in the VapXD Toxin-Antitoxin System. Structure. 2021;29(2):139-50. doi:10.1016/j.str.2020.10.002
50. Herraiz CG. Anticuerpos monoclonals frente a PCSK9: del desarrollo básico a la clínica. Clín Investig Arteroscler. 2016;28(Suppl 2):14-21. doi:10.1016/S0214-9168(16)30166-8
51. Le Basle Y, Chennell P, Tokhadze N, Astier A, Sautou V. Physicochemical Stability of Monoclonal Antibodies: A Review. J Pharm Sci. 2020;109(1):169-90. doi:10.1016/j.xphs.2019.08.009
52. Ying T, Gong R, Ju TW, Prabakaran P, Dimitrov DS. Engineered Fc based antibody domains and fragments as novel scaffolds. Biochim Biophys Acta. 2014;1844(11):1977-82. doi:10.1016/j.bbapap.2014.04.018
53. Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317-27. doi:10.1038/nri2744
54. Morisson SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984;81(21):6851-5. doi:10.1073/pnas.81.21.6851
55. Mitra S, Tomar PC. Hybridoma technology; advancements, clinical significance, and future aspects. J Genet Eng Biotechnol. 2021;19(1):159. doi:10.1186/s43141-021-00264-6
56. Nicholson LB. The immune system. Essays Biochem. 2016;60(3):275-301. doi:10.1042/ebc20160017
57. Bayer V. An Overview of Monoclonal Antibodies. Semin Oncol Nurs. 2019;35(5):150927. doi:10.1016/j.soncn.2019.08.006
58. Buss NA, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12(5):615-22. doi:10.1016/j.coph.2012.08.001
59. Kumar R, Parray HA, Shrivastava T, Sinha S, Luthra K. Phage display antibody libraries: A robust approach for generation of recombinant human monoclonal antibodies. Int J Biol Macromol. 2019;135:907-18. doi:10.1016/j.ijbiomac.2019.06.006
60. Sidhu SS. Antibodies for all: The case for genome-wide affinity reagents. FEBS Letters. 2012;586(17):2778-9. doi:10.1016/j.febslet.2012.05.044
61. Nielsen UB, Marks JD. Internalizing antibodies and targeted cancer therapy: direct selection from phage display libraries. Pharm Sci Technol Today. 2000;3(8):282-91. doi:10.1016/s1461-5347(00)00280-7
62. Rodgers KR, Chou RC. Therapeutic monoclonal antibodies and derivatives: Historical perspectives and future directions. Biotechnol Adv. 2016;34(6):1149-58. doi:10.1016/j.biotechadv.2016.07.004
63. Lonberg N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol. 2008;20(4):450-9. doi:10.1016/j.coi.2008.06.004
64. Shukla AA, Thömmes J. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol. 2010;28(5):253-61. doi:10.1016/j.tibtech.2010.02.001
65. Dodd R, Schofield DJ, Wilkinson T, Britton ZT. Generating therapeutic monoclonal antibodies to complex multi-spanning membrane targets: Overcoming the antigen challenge and enabling discovery strategies. Methods. 2020;180:111-26. doi:10.1016/j.ymeth.2020.05.006
66. Fliedl L, Grillari J, Grillari-Voglauer R. Human cell lines for the production of recombinant proteins: on the horizon. N Biotechnol. 2015;32(6):673-9. doi:10.1016/j.nbt.2014.11.005
67. Li F, Vijayasankaran N, Shen AY, Kiss R, Amanullah A. Cell culture processes for monoclonal antibody production. mAbs. 2010;2(5):466-79. doi:10.4161/mabs.2.5.12720
68. Lanter C, Lev M, Cao L, Loladze V. Rapid Intact mass based multi-attribute method in support of mAb upstream process development. J Biotechnol. 2020;314-315:63-70. doi:10.1016/j.jbiotec.2020.04.001
69. Jain E, Kumar A. Upstream processes in antibody production: Evaluation of critical parameters. Biotechnol Adv. 2008;26(1):46-72. doi:10.1016/j.biotechadv.2007.09.004
70. Chahar DS, Ravindran S, Pisal SS. Monoclonal antibody purification and its progression to commercial scale. Biologicals. 2020;63:1-13. doi:10.1016/j.biologicals.2019.09.007
71. Chon JH, Zarbis-Papastoitsis G. Advances in the production and downstream processing of antibodies. N Biotechnol. 2011;28(5):458-63. doi:10.1016/j.nbt.2011.03.015
72. Vasiljevic S, Beale EV, Bonomelli C, Easthope IS, Pritchard LK, Seabright GE, et al. Redirecting adenoviruses to tumour cells using therapeutic antibodies: Generation of a versatile human bispecific adaptor. Mol Immunol. 2015;68(2, Part A):234-43. doi:10.1016/j.molimm.2015.08.014
73. Hnasko RM, McGarvey JA. Affinity Purification of Antibodies. Methods Mol Biol. 2015;1318:29-41. doi:10.1007/978-1-4939-2742-5_3
74. Urmann M, Graalfs H, Joehnck M, Jacob LR, Frech C. Cation-exchange chromatography of monoclonal antibodies: characterisation of a novel stationary phase designed for production-scale purification. MAbs. 2010;2(4):395-404. doi:10.4161/mabs.12303
75. Ladner Y, Mas S, Coussot G, Bartley K, Montels J, Morel J, et al. Integrated microreactor for enzymatic reaction automation: An easy step toward the quality control of monoclonal antibodies. J Chromatogr A. 2017;1528:83-90. doi:10.1016/j.chroma.2017.10.066
76. Barcelona PF, Galan A, Nedev H, Jian Y, Sarunic MV, Saragovi HU. The route of administration influences the therapeutic index of an anti-proNGF neutralizing mAb for experimental treatment of Diabetic Retinopathy. PloS One. 2018;13(6):e0199079. doi:10.1371/journal.pone.0199079
77. VanderVeen DK, Cataltepe SU. Anti-vascular endothelial growth factor intravitreal therapy for retinopathy of prematurity. Semin Perinatol. 2019;43(16):375-80. doi:10.1053/j.semperi.2019.05.011
78. Franco CJV, Monsalve P, Martínez GIS, Rivera A, Zuluaga L, Duran C, et al. Uveítis y terapia anti-TNF. Rev Colomb Reumatol. 2011;18(1):42-54.
79. Tolentino M. Systemic and Ocular Safety of Intravitreal Anti-VEGF Therapies for Ocular Neovascular Disease. Surv Ophthalmol. 2011;56(2):95-113. doi:10.1016/j.survophthal.2010.08.006
80. Fogli S, Del Re M, Rofi E, Posarelli C, Figus M, Danesi R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye. 2018;32(6):1010-20. doi:10.1038/s41433-018-0021-7
81. Wu Q, Sun X, Zheng G. VEGF overexpression is associated with optic nerve involvement and differentiation of retinoblastoma: A PRISMA-compliant meta-analysis. Medicine. 2018;97(51):e13753. doi:10.1097/MD.0000000000013753
82. Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2(12):1097-105. doi:10.1177/1947601911423031
83. Durrani K, Kempen JH, Ying GS, Kacmaz RO, Artornsombudh P, Rosenbaum JT, et al. Adalimumab for Ocular Inflammation. Ocul Immunol Inflamm. 2017;25(3):405-12. doi:10.3109/09273948.2015.1134581
84. Ono M, Horita S, Sato Y, Nomura Y, Iwata S, Nomura N. Structural basis for tumor necrosis factor blockade with the therapeutic antibody golimumab. Protein Sci. 2018;27(6):1038-46. doi:10.1002/pro.3407
85. Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in Patients with Active Noninfectious Uveitis. N Engl J Med. 2016;375(10):932-43. doi:10.1056/NEJMoa1509852
86. Kruh JN, Yang P, Suelves AM, Foster CS. Infliximab for the Treatment of Refractory Noninfectious Uveitis: A Study of 88 Patients with Long-term Follow-up. Ophthalmology. 2014;121(1):358-64. doi:10.1016/j.ophtha.2013.07.019
87. Markomichelakis N, Delicha E, Masselos S, Sfikakis PP. Intravitreal Infliximab for Sight-Threatening Relapsing Uveitis in Behçet Disease: A Pilot Study in 15 Patients. Am J Ophthalmol. 2012;154(3):534–41. doi:10.1016/j.ajo.2012.03.035
88. Khalili H, Lee RW, Khaw PT, Brocchini S, Dick AD, Copland DA. An anti-TNF-α antibody mimetic to treat ocular inflammation. Sci Rep. 2016;6:36905. doi:10.1038/srep36905
89. Nesbitt A, Fossati G, Bergin M, Stephens P, Stephens S, Foulkes R, et al. Mechanism of Action of Certolizumab Pegol (CDP870): In Vitro Comparison with Other Anti-tumor Necrosis Factor α agents. Inflamm Bowel Dis. 2007;13(11):1323-32. doi:10.1002/ibd.20225
90. Sharon Y, Chu DS. Certolizumab pegol - Tumor necrosis factor inhibitor for refractory uveitis. Am J Ophthalmol Case Rep. 2020;18:100633. doi:10.1016/j.ajoc.2020.100633
91. Tosi GM, Sota J, Vitale A, Rigante D, Emmi G, Lopalco G, et al. Efficacy and safety of certolizumab pegol and golimumab in the treatment of non-infectious uveitis. Clin Exp Rheumatol. 2019;37(4):680-3.
92. Touzani F, Geers C, Pozdzik A. Intravitreal Injection of Anti-VEGF Antibody Induces Glomerular Endothelial Cells Injury. Case Rep Nephrol. 2019;2019:2919080. doi:10.1155/2019/2919080
93. Ferrer LG, López MR, Santana YM, Hernández MC, Miniet EP, Reydmond KG. Estrategias en el tratamiento de la retinopatía diabética. Rev Cubana Olftalmol. 2018;31(1):90-9.
94. Perea JRA, Layana AG. Ranibizumab versus bevacizumab. Pharmacological considerations. Arch Soc Esp Oftalmol. 2012;87(Suppl 1):3-9. doi:10.1016/S0365-6691(12)70046-1
95. Wu AL, Wu WC. Anti-VEGF for ROP and Pediatric Retinal Diseases. Asia Pac J Ophthalmol. 2018;7(3):145-51. doi:10.22608/APO.201837
96. Ha JY, Lee TH, Sung MS, Park SW. Efficacy and Safety of Intracameral Bevacizumab for Treatment of Neovascular Glaucoma. Korean J Ophthalmol. 2017;31(6):538-47. doi:10.3341/kjo.2017.0017
97. Jiang S, Park C, Barner JC. Ranibizumab for age-related macular degeneration: a meta-analysis of dose effects and comparison with no anti-VEGF treatment and bevacizumab. J Clin Pharm Therap. 2014;39(3):234-9. doi:10.1111/jcpt.12146
98. Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;8(8):CD005139. doi:10.1002/14651858.CD005139.pub3
99. Leclercq M, Desbois AC, Domont F, maalouf G, Touhami S, Cacoub P, et al. Biotherapies in Uveitis. J Clin Med. 2020;9(11):3599. doi:10.3390/jcm9113599
100. Haug SJ, Hien DL, Uludag G, Ngoc TTT, Lajevardi S, Halim MS, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi:10.1016/j.ajoc.2020.100680
101. Nguyen QD, Das A, Do DV, Dugel PU, Gomes A, Holz FG, et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020;127(7):963-76. doi:10.1016/j.ophtha.2019.12.031
102. Karasavvidou EM, Tranos P, Panos GD. Brolucizumab for the Treatment of Degenerative Macular Conditions: A Review of Clinical Studies. Drug Des Devel Ther. 2022;16:2659-80. doi:10.2147/dddt.s378450
103. Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13(9):1972-88. doi:10.1080/21645515.2017.1316909
104. Atienza-Mateo B, Calvo-Río V, Beltrán E, Martínez-Costa L, Valls-Pascual E, Hernández-Garfella M, et al. Anti-interleukin 6 receptor tocilizumab in refractory uveitis associated with Behçet's disease: multicentre retrospective study. Rheumatology. 2018;57(5):856-64. doi:10.1093/rheumatology/kex480
105. Strohbehn GW, Heiss BL, Rouhani SJ, Trujillo JA, Yu J, Kacew AJ, et al. COVIDOSE: A Phase II Clinical Trial of Low-Dose Tocilizumab in the Treatment of Noncritical COVID-19 Pneumonia. Clin Pharmacol Ther. 2021;109(3):688-96. doi:10.1002/cpt.2117
106. Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn´s Disease. N Engl J Med. 2016;375(20):1946-60. doi:10.1056/NEJMoa1602773
107. Pepple KL, Lin P. Targeting Interleukin-23 in the Treatment of Noninfectious Uveitis. Ophthalmology. 2018;125(12):1977-83. doi:10.1016/j.ophtha.2018.05.014
108. Shirley M. Faricimab: First Approval. Drugs. 2022;82(7):825-30. doi:10.1007/s40265-022-01713-3
109. Chakravarthy U, Bailey C, Brown D, Campochiaro P, Chittum M, Csaky K, et al. Phase I Trial of Anti-Vascular Endothelial Growth Factor/Antiangiopoietin 2 Bispecific Antibody RG7716 for Neovascular Age-Related Macular Degeneration. Ophthalmol Retina. 2017;1(6):474-85. doi:10.1016/j.oret.2017.03.003
110. Sahni J, Patel SS, Dugel PU, Khanani AM, Jhaveri CD, Wykoff CC, et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology. 2019;126(8):1155-70. doi:10.1016/j.ophtha.2019.03.023
111. Sharma S, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Faricimab: expanding horizon beyond VEGF. Eye. 2020;34(5):802-4. doi:10.1038/s41433-019-0670-1
Authors
Copyright (c) 2022 Catalina Ayón, Daniel Castán, Adrián Mora, Dunia Naranjo, Francini Obando, Juan José Mora

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.