Antioxidant Activity of n-hexane and Etil Acetate Fractions of Bangkal (Nauclea subdita (Korth.) Steud.) Leaves
Abstract
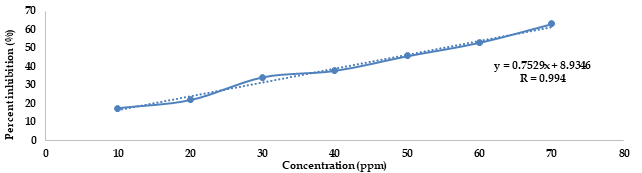
Bangkal (Nauclea subdita (Korth.) Steud.) is a tropical plant belonging to the Rubiaceae family, commonly found in South Kalimantan. This plant is one of the plants that has efficacy as a medicinal plant. This study aimed to quantitatively identify secondary metabolites and antioxidant activity in the n-hexane and ethyl acetate fractions of N. subdita leaves. The method of identification of secondary metabolites using the test tube. Antioxidant activity using the DPPH method based on IC50 value. The results of identifying secondary metabolites in the n-hexane fraction of N. subdita leaves contain alkaloids, flavonoids, steroids, and phenolic compounds, while the ethyl acetate fraction of N. subdita leaves contain alkaloids, flavonoids, steroids, tannins, saponins, and phenolics. The results of the antioxidant activity test of the n-hexane fraction and the ethyl acetate fraction of the leaves of N. subdita showed IC50 values of 229.61178±3.65919 and 54.54296±0.02236 ppm, respectively. Based on the IC50 value, the n-hexane fraction of N. subdita leaves had weak antioxidant activity, and the ethyl acetate fraction of N. subdita leaves had strong antioxidant activity.
Full text article
References
2. Roy A, Khan A, Ahmad I, Alghamdi S, Rajab BS, Babaghith AO, et al. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res Int. 2022;2022:5445291. doi:10.1155/2022/5445291
3. Zehiroglu C, Sarikaya SBO. The importance of antioxidants and place in today's scientific and technological studies. J Food Sci Technol. 2019;56(11):4757-74. doi:10.1007/s13197-019-03952-x
4. Flieger J, Flieger W, Baj J, Maciejewski R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials. 2021;14(15):4135. doi:10.3390/ma14154135
5. Manessis G, Kalogianni AI, Lazou T, Moschovas M, Bossis I, Gelasakis AI. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants. 2020;9(12):1215. doi:10.3390/antiox9121215
6. Asmiyarti NI, Wibowo MA. Uji Aktivitas Antioksidan Metode DPPH dan Uji Sitotoksik Metode BSLT pada Ekstrak Metanol Daun Bongkal (Nuaclea subdita (Korth) Steud). J Kimia Khatulistiwa. 2014;3(1):58-62.
7. Aisiah S. Potensi Tumbuhan Bangkal (Nauclea Orientalis) untuk Pengendalian Bakteri Aeromonas hydrophila. Fish Sci. 2016;2(4):166-77. doi:10.20527/fs.v2i4.1172
8. Wardhani RRAAK, Akhyar O. Analisis Skrining Fitokimia, Aktivitas Antioksidan dan Antibakteri Propionibacterium acnes Ekstrak Etanol Kulit Batang dan Daun Tanaman Bangkal (Nuclea subdita). J Ilmiah Berkala Sains Terapan Kimia. 2018;12(2):64-75. doi:10.20527/jstk.v12i2.4956
9. Abubakar AR, Haque M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J Pharm Bioallied Sci. 2020;12(1):1-10. doi:10.4103/jpbs.jpbs_175_19
10. Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13:20. doi:10.1186/s13020-018-0177-x
11. Dubale S, Kebebe D, Zeynudin A, Abdissa N, Suleman S. Phytochemical Screening and Antimicrobial Activity Evaluation of Selected Medicinal Plants in Ethiopia. J Exp Pharmacol. 2023;15:51-62. doi:10.2147/jep.s379805
12. Muthia R, Saputri R, Verawati SA. Uji Aktivitas Antioksidan Ekstrak Etanol Kulit Buah Mundar (Garcinia forbesii King.) Menggunakan Metode DPPH (2,2-diphenyl-1-picrylhydrazil). J Pharmascience. 2019;6(1):74-82. doi:10.20527/jps.v6i1.6079
13. Itam A, Wati MS, Agustin V, Sabri N, Jumanah RA, Efdi M. Comparative Study of Phytochemical, Antioxidant, and Cytotoxic Activities and Phenolic Content of Syzygium aqueum (Burm. f. Alston f.) Extracts Growing in West Sumatera Indonesia. ScientificWorldJournal. 2021;2021:5537597. doi:10.1155/2021/5537597
14. Abdelmohsen UR, Sayed AM, Elmaidomy AH. Natural Products’ Extraction and Isolation-Between Conventional and Modern Techniques. Front Nat Prod. 2022;1:873808. doi:10.3389/fntpr.2022.873808
15. Silaban H. The Effect of Various Concentrations of Ethanol Extract of the Leaves of Paederia foetida L. on the Growth of Escherichia Coli Bacteria. J Drug Deliv Ther. 2021;11(6):61-7. doi:10.22270/jddt.v11i6.5037
16. Thavamoney N, Sivanadian L, Tee LH, Khoo HE, Prasad KN, Kong KW. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J Food Sci Technol. 2018;55(7):2523-32. doi:10.1007/s13197-018-3170-6
17. Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lighfoot DA. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants. 2017;6(4):42. doi:10.3390/plants6040042
18. Friesen JB, McAlpine JB, Chen SN, Pauli GF. Countercurrent Separation of Natural Products: An Update. J Nat Prod. 2015;78(7):1765-96. doi:10.1021/np501065h
19. Hayat J, Akodad M, Moumen A, Baghour M, Skalli A, Ezrari S, et al. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon. 2020;6(11):e05609. doi:10.1016/j.heliyon.2020.e05609
20. Autor E, Cornejo A, Bimbela F, Maisterra M, Gandia LM, Martínez-Merino V. Extraction of Phenolic Compounds from Populus Salicaceae Bark. Biomolecules. 2022;12(4):539. doi:10.3390/biom12040539
21. Syarifah AL, Retnowati R, Soebiantoro. Characterization of Secondary Metabolites Profile of Flavonoid from Salam Leaves (Eugenia polyantha) Using TLC and UVSpectrophotometry. Pharm Sci Res. 2019;6(3):155-63. doi:10.7454/psr.v6i3.4219
22. Hancu G, Simon B, Kelemen H, Rusu A, Mircia E, Gyeresi A. Thin layer chromatographic analysis of Beta-lactam antibiotics. Adv Pharm Bull. 2013;3(2):367-71. doi:10.5681/apb.2013.059
23. Sherma J, Rabel F. A Review of Thin Layer Chromatography Methods for Determination of Authenticity of Foods and Dietary Supplements. J Liq Chromatogr Relat Technol. 2018;41(10):645-57. doi:10.1080/10826076.2018.1505637
24. Pratiwi RA, Nandiyanto ABD. How to Read and Interpret UV-Vis Spectrophotometric Results in Determining the Structure of Chemical Compounds. Indones J Educ Res Technol. 2022;2(1):1-20. doi:10.17509/ijert.v2i1.35171
25. Sari AK, Ayati R. Penentuan Aktivitas Antioksidan Ekstrak Etanol Daun Jeruk Purut (Citrus hystrix D. C) dengan Metode DPPH (1,1-diphenyl-2- picrylhydrazyl). J Curr Pharm Sci. 2018;1(2):69-74.
26. Molyneux P. The Use of The Stable Free Radical Diphenylpicryl-Hydrazyl (DPPH) for Estimating Antioxidant Activity. Sonklanakarin j Sci Technol. 2004;26(2):211-9.
27. Shahidi F, Zhong Y. Measurement of antioxidant activity. J Funct Foods. 2015;18(B):757-81. doi:10.1016/j.jff.2015.01.047
28. Wolosiak R, Drużyńska B, Derewiaka D, Piecyk M, Majewska E, Ciecierska M, et al. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays-A Practical Approach. Molecules. 2021;27(1):50. doi:10.3390/molecules27010050
29. Adawiyah R, Rizki MI. Aktivitas Antioksidan Ekstrak Etanol Akar Kalakai (Stenochlaena palustris Bedd) Asal Kalimantan Tengah. J Pharmascience. 2018;5(1):71-7.10.20527/jps.v5i1.5788
30. Li Z, Moalin M, Zhang M, Vervoort L, Hursel E, Mommers A, et al. The Flow of the Redox Energy in Quercetin during Its Antioxidant Activity in Water. Int J Mol Sci. 2020;21(17):6015. doi:10.3390/ijms21176015
31. Maesaroh K, Kurnia D, Anshori JA. Perbandingan Metode Uji Aktivitas Antioksidan DPPH, FRAP dan FIC Terhadap Asam Askorbat, Asam Galat dan Kuersetin. Chim Nat Acta. 2018;6(2):93-100. doi:10.24198/cna.v6.n2.19049
32. Rizki MI, Nurlely, Fadlilaturrahmah, Ma’shumah. Aktivitas Antioksidan Ekstrak Etanol Daun Cempedak (Artocarpus integer), Nangka (Artocarpus heterophyllus), dan Tarap (Artocarpus odoratissimus) Asal Kalimantan Selatan. J Curr Pharm Sci. 2021;4(2):367-72.
Authors
Copyright (c) 2023 Arnida Arnida, Al Madani, Sutomo Sutomo

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.