Formulation and Evaluation of Soursop (Annona muricata) Leaf Extract Nanoemulgel Against Propionibacterium acnes
Abstract
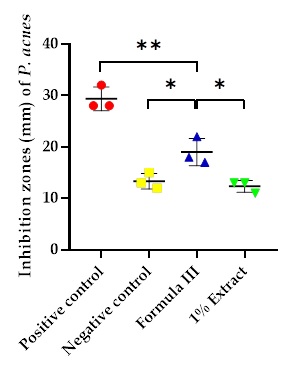
Annona muricata (soursop) leaves are rich in antimicrobial compounds such as flavonoids, alkaloids, tannins, saponins, and phenols. This study aimed to develop a nanoemulgel formulation incorporating A. muricata leaf ethanol extract to enhance its efficacy against Propionibacterium acnes, a bacterium associated with acne vulgaris. Four nanoemulgel formulations containing varying concentrations of the extract (0%, 0.5%, 0.7%, and 1%) were prepared and evaluated for their physical properties (organoleptic, homogeneity, pH, spreadability, and viscosity) and stability through freeze-thaw cycles. The formulation with the highest extract concentration (Formula III) was selected for further characterization (particle size, morphology, and zeta potential) and antimicrobial testing against P. acnes. All formulations met the established physical property and stability criteria. Formula III exhibited a particle size of 20.5 nm and a zeta potential of 9.8 mV, indicating a stable nanoemulsion with well-dispersed particles. Antimicrobial testing revealed that Formula III demonstrated a strong inhibitory effect against P. acnes, with an average inhibition zone of 19.00 mm. These findings suggest that A. muricata leaf extract-loaded nanoemulgel has the potential to be a promising topical formulation for acne treatment. Further research is warranted to optimize the formulation and evaluate its efficacy in clinical settings.
Full text article
References
2. Ahmad J, Gautam A, Komath S, Bano M, Garg A, Jain K. Topical Nano-emulgel for Skin Disorders: Formulation Approach and Characterization. Recent Pat Antiinfect Drug Discov. 2018;14(1):36–48. DOI: 10.2174/1574891x14666181129115213; PMID: 30488798
3. Yang JH, Yoon JY, Kwon HH, Min S, Moon J, Suh DH. Seeking new acne treatment from natural products, devices and synthetic drug discovery. Dermatoendocrinol. 2017;9(1):e1356520. DOI: 10.1080/19381980.2017.1356520; PMCID: PMC5821150; PMID: 29484092
4. Hasmila I, Natsir H, Soekamto NH. Phytochemical analysis and antioxidant activity of soursop leaf extract (Annona muricata Linn.). J Phys Conf Ser. 2019;1341(3):032027. DOI: 10.1088/1742-6596/1341/3/032027
5. Esparza LMA, Montalvo-González E. Bioactive Compounds of Soursop (Annona muricata L.) Fruit. In: Mérillon JM, Ramawat KG, editors. Reference Series in Phytochemistry. Cham: Springer; 2020. 175–89. DOI: 10.1007/978-3-030-30182-8_8
6. Rarassari MA, Maftuch HN. Phytochemicals and Antibacterial Activities of Soursop Leaf (Annona muricata) against Edwardsiella tarda (In Vitro). J Life Sci Biomed. 2016;6(1):6–9.
7. Primasari A, Nasution M, Arbi NH, Sari DP, Basyuni M. The effectiveness of soursop leaf extract against growth of aggregatibacter actinomycetemcomitans atcc® 6514TM in vitro. Asian J Pharm Clin Res. 2018;11:411–5. DOI: 10.22159/ajpcr.2018.v11i12.28435
8. Coria-Téllez AV., Montalvo-Gónzalez E, Yahia EM, Obledo-Vázquez EN. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem. 2018;11(5):662–91. DOI: 10.1016/j.arabjc.2016.01.004
9. Mulyanti D, Rismawati, E, Maulana IT, Febriani D, Dewi YN. Uji Aktivitas Antibakteri Ekstrak Etanol Daun Sirsak (Annona muricata L.) pada Bakteri Propionibacterium acnes, Staphylococcus aureus, dan Staphylococcus epidermidis. Prosiding SNaPP Kesehatan Kedokteran Kebidanan Keperawatan Farmasi Psikologi. 2015;1(1):325–30.
10. Lal DK, Kumar B, Saeedan AS, Ansari MN. An Overview of Nanoemulgels for Bioavailability Enhancement in Inflammatory Conditions via Topical Delivery. Pharmaceutics. 2023;15(4):1187. DOI: 10.3390/pharmaceutics15041187; PMCID: PMC10145625; PMID: 37111672
11. Donthi MR, Munnangi SR, Krishna KV, Saha RN, Singhvi G, Dubey SK. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics. 2023;15(1):164. DOI: 10.3390/pharmaceutics15010164; PMCID: PMC9863395; PMID: 36678794
12. Duarte J, Sharma A, Sharifi E, Damiri F, Berrada M, Khan MA, et al. Topical delivery of nanoemulsions for skin cancer treatment. Appl Mater Today. 2023;35:102001. DOI: 10.1016/j.apmt.2023.102001
13. Arora R, Aggarwal G, Harikumar SL, Kaur K. Nanoemulsion Based Hydrogel for Enhanced Transdermal Delivery of Ketoprofen. Adv Pharm. 2014;1:468456. DOI: 10.1155/2014/468456
14. Tayeb HH, Felimban R, Almaghrabi S, Hasaballah N. Nanoemulsions: Formulation, characterization, biological fate, and potential role against COVID-19 and other viral outbreaks. Colloid Interface Sci Commun. 2021;45:100533. DOI: 10.1016/j.colcom.2021.100533; PMCID: PMC8526445; PMID: 34692429
15. Akhter A, Shirazi JH, Khan HMS, Hussain MD, Kazi M. Development and evaluation of nanoemulsion gel loaded with bioactive extract of Cucumis melo var. agrestis: A novel approach for enhanced skin permeability and antifungal activity. Heliyon. 2024;10(15):e35069. DOI: 10.1016/j.heliyon.2024.e35069
16. Ullah N, Amin A, Farid A, Selim S, Rashid SA, Aziz MI, et al. Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections. Gels. 2023;9(3):252. DOI: 10.3390/gels9030252; PMCID: PMC10048686; PMID: 36975701
17. Eid AM, El-Enshasy HA, Aziz R, Elmarzugi NA. Preparation, characterization and anti-inflammatory activity of Swietenia macrophylla nanoemulgel. J Nanomedicine Nanotechnol. 2014;5(2):1-10. DOI: 10.4172/2157-7439.1000190
18. Ali SM, Yosipovitch G. Skin pH: From basic science to basic skin care. Acta Derm Venereol. 2013;93(3):261–7. DOI: 10.2340/00015555-1531; PMID: 23322028
19. Gadkari PN, Patil PB, Saudagar RB. Formulation, development and evaluation of topical nanoemulgel of tolnaftate. J Drug Deliv Ther. 2019;9(2-S):208–13. DOI: 10.22270/jddt.v9i2-s.2495
20. Sulistyo H, Kurniawan DW, Rujito L. Biochemical and histopathological effects of green tea nanoparticles in ironized mouse model. Res Pharm Sci. 2017;12(2):99–106. DOI: 10.4103/1735-5362.202448; PMCID: PMC5385734; PMID: 28515762
21. Kurniawan DW, Booijink R, Pater L, Wols I, Vrynas A, Storm G, et al. Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. J Control Release. 2020;328:640–52. DOI: 10.1016/j.jconrel.2020.09.041
22. Gurpreet K, Singh SK. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J Pharm Sci. 2018;80(5):781–9. DOI: 10.4172/pharmaceutical-sciences.1000422
23. Clogston JD, Patri AK. Zeta potential measurement. Methods Mol Biol. 2011;697:63–70. DOI: 10.1007/978-1-60327-198-1_6; PMID: 21116954
24. Ismanto DS, Eliyasmi R, Osman D. Penambahan Ekstrak Daun Sırsak Terhadap Mınuman Instan darı Buah Sırsak (Annona muricata, L). In: Prosiding Seminar Lokakarya Nasional FKPT-TPI. Pekanbaru: Universitas Riau; 2014. 211–9.
25. Preeti, Sambhakar S, Malik R, Bhatia S, Al Harrasi A, Rani C, et al. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica. 2023;2023:6640103. DOI: 10.1155/2023/6640103; PMCID: PMC10625491; PMID: 37928749
26. Sumantri I, Hermawan GP, Laksono H. Ekstraksi Daun Sirsak (Annona muricata L.) Menggunakan Pelarut Etanol. Majalah Ilmiah Momentum. 2014;10(1):34–7. DOI: 10.36499/jim.v10i1.961
27. Sengupta P, Chatterjee B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int J Pharm. 2017;526(1-2):353–65. DOI: 10.1016/j.ijpharm.2017.04.068; PMID: 28461261
28. Sheskey PJ, Cook WG, Cable CG. Handbook of Pharmaceutical Excipients, 8th ed. London: Pharmaceutical Press; 2017. 468.
29. Iinuma K, Noguchi N, Nakaminami H, Sasatsu M, Nishijima S, Tsuboi I. Susceptibility of Propionibacterium acnes isolated from patients with acne vulgaris to zinc ascorbate and antibiotics. Clin Cosmet Investig Dermatol. 2011;4:161-5. DOI: 10.2147/ccid.s23840; PMCID: PMC3208449; PMID: 22087070
Authors
Copyright (c) 2024 Nabila Ikramina, Rehana Rehana, Rahmad Aji Prasetya, Dhadhang Wahyu Kurniawan

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Authors continue to retain the copyright to the article if the article is published in the Borneo Journal of Pharmacy. They will also retain the publishing rights to the article without any restrictions.
Authors who publish in this journal agree to the following terms:
- Any article on the copyright is retained by the author(s).
- The author grants the journal the right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share work with an acknowledgment of the work authors and initial publications in this journal.
- Authors can enter into separate, additional contractual arrangements for the non-exclusive distribution of published articles (e.g., post-institutional repository) or publish them in a book, with acknowledgment of their initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their websites) prior to and during the submission process. This can lead to productive exchanges and earlier and greater citations of published work.
- The article and any associated published material are distributed under the Creative Commons Attribution-ShareAlike 4.0 International License.